New neurons in old brains: implications of age in the analysis of neurogenesis in post-mortem tissue
- PMID: 35501905
- PMCID: PMC9063342
- DOI: 10.1186/s13041-022-00926-7
New neurons in old brains: implications of age in the analysis of neurogenesis in post-mortem tissue
Abstract
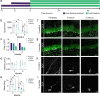
Adult neurogenesis, the proliferation and integration of newly generated neurons, has been observed in the adult mammalian hippocampus of many species. Numerous studies have also found adult neurogenesis in the human hippocampus, but several recent high-profile studies have suggested that this process is considerably reduced in humans, occurring in children but not in adults. In comparison, rodent studies also show age-related decline but a greater degree of proliferation of new neurons in adult animals. These differences may represent biological species differences or could alternatively be explained by methodological differences in tissue handling and fixation. Here, we examine whether differences in the post-mortem interval between death and tissue fixation might impact subsequent detection of adult neurogenesis due to increased tissue degradation. Because there are fewer new neurons present in older subjects to begin with we hypothesized that, subject age might interact significantly with post-mortem interval in the detection of adult neurogenesis. We analyzed neurogenesis in the hippocampus of rats that were either perfusion-fixed or the brains extracted and immersion-fixed at various post-mortem intervals. We observed an interaction between animal age and the time delay between death and tissue fixation. While similar levels of neurogenesis were observed in young rats regardless of fixation, older rats had significantly fewer labeled neurons when fixation was not immediate. Furthermore, the morphological detail of the labeled neurons was significantly reduced in the delayed fixation conditions at all ages. This study highlights critical concerns that must be considered when using post-mortem tissue to quantify adult neurogenesis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med Springer Science and Business Media LLC. 1998;4:1313–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

