Alzheimer risk gene product Pyk2 suppresses tau phosphorylation and phenotypic effects of tauopathy
- PMID: 35501917
- PMCID: PMC9063299
- DOI: 10.1186/s13024-022-00526-y
Alzheimer risk gene product Pyk2 suppresses tau phosphorylation and phenotypic effects of tauopathy
Erratum in
-
Correction: Alzheimer risk gene product Pyk2 suppresses tau phosphorylation and phenotypic effects of tauopathy.Mol Neurodegener. 2022 Aug 30;17(1):56. doi: 10.1186/s13024-022-00557-5. Mol Neurodegener. 2022. PMID: 36038879 Free PMC article. No abstract available.
Abstract
Background: Genetic variation at the PTK2B locus encoding the protein Pyk2 influences Alzheimer's disease risk. Neurons express Pyk2 and the protein is required for Amyloid-β (Aβ) peptide driven deficits of synaptic function and memory in mouse models, but Pyk2 deletion has minimal effect on neuro-inflammation. Previous in vitro data suggested that Pyk2 activity might enhance GSK3β-dependent Tau phosphorylation and be required for tauopathy. Here, we examine the influence of Pyk2 on Tau phosphorylation and associated pathology.
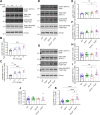
Methods: The effect of Pyk2 on Tau phosphorylation was examined in cultured Hek cells through protein over-expression and in iPSC-derived human neurons through pharmacological Pyk2 inhibition. PS19 mice overexpressing the P301S mutant of human Tau were employed as an in vivo model of tauopathy. Phenotypes of PS19 mice with a targeted deletion of Pyk2 expression were compared with PS19 mice with intact Pyk2 expression. Phenotypes examined included Tau phosphorylation, Tau accumulation, synapse loss, gliosis, proteomic profiling and behavior.
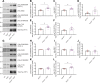
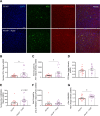
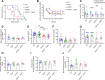
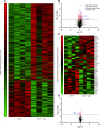
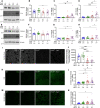
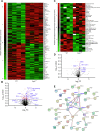
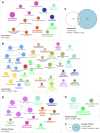
Results: Over-expression experiments from Hek293T cells indicated that Pyk2 contributed to Tau phosphorylation, while iPSC-derived human neuronal cultures with endogenous protein levels supported the opposite conclusion. In vivo, multiple phenotypes of PS19 were exacerbated by Pyk2 deletion. In Pyk2-null PS19 mice, Tau phosphorylation and accumulation increased, mouse survival decreased, spatial memory was impaired and hippocampal C1q deposition increased relative to PS19 littermate controls. Proteomic profiles of Pyk2-null mouse brain revealed that several protein kinases known to interact with Tau are regulated by Pyk2. Endogenous Pyk2 suppresses LKB1 and p38 MAPK activity, validating one potential pathway contributing to increased Tau pathology.
Conclusions: The absence of Pyk2 results in greater mutant Tau-dependent phenotypes in PS19 mice, in part via increased LKB1 and MAPK activity. These data suggest that in AD, while Pyk2 activity mediates Aβ-driven deficits, Pyk2 suppresses Tau-related phenotypes.
Keywords: Alzheimer’s disease; C1q; Fronto-temporal dementia; PTK2B; Pyk2; Tauopathy.
© 2022. The Author(s).
Conflict of interest statement
None.
Figures


References
-
- 2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17:327–406. - PubMed
-
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, DeStafano AL, Bis JC, Beecham GW, Grenier-Boley B, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nat Genet. 2013;45:1452–1458. doi: 10.1038/ng.2802. - DOI - PMC - PubMed
-
- Beecham GW, Hamilton K, Naj AC, Martin ER, Huentelman M, Myers AJ, Corneveaux JJ, Hardy J, Vonsattel JP, Younkin SG, et al. Genome-wide association meta-analysis of neuropathologic features of Alzheimer's disease and related dementias. Plos Genet. 2014;10:e1004606. doi: 10.1371/journal.pgen.1004606. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

