Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain
- PMID: 35501923
- PMCID: PMC9063061
- DOI: 10.1186/s40168-022-01243-w
Fecal microbiota transfer between young and aged mice reverses hallmarks of the aging gut, eye, and brain
Abstract
Background: Altered intestinal microbiota composition in later life is associated with inflammaging, declining tissue function, and increased susceptibility to age-associated chronic diseases, including neurodegenerative dementias. Here, we tested the hypothesis that manipulating the intestinal microbiota influences the development of major comorbidities associated with aging and, in particular, inflammation affecting the brain and retina.
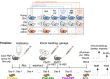
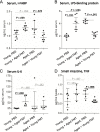
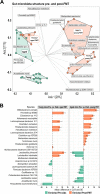
Methods: Using fecal microbiota transplantation, we exchanged the intestinal microbiota of young (3 months), old (18 months), and aged (24 months) mice. Whole metagenomic shotgun sequencing and metabolomics were used to develop a custom analysis workflow, to analyze the changes in gut microbiota composition and metabolic potential. Effects of age and microbiota transfer on the gut barrier, retina, and brain were assessed using protein assays, immunohistology, and behavioral testing.
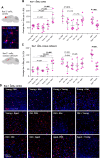
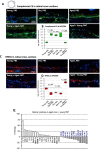
Results: We show that microbiota composition profiles and key species enriched in young or aged mice are successfully transferred by FMT between young and aged mice and that FMT modulates resulting metabolic pathway profiles. The transfer of aged donor microbiota into young mice accelerates age-associated central nervous system (CNS) inflammation, retinal inflammation, and cytokine signaling and promotes loss of key functional protein in the eye, effects which are coincident with increased intestinal barrier permeability. Conversely, these detrimental effects can be reversed by the transfer of young donor microbiota.
Conclusions: These findings demonstrate that the aging gut microbiota drives detrimental changes in the gut-brain and gut-retina axes suggesting that microbial modulation may be of therapeutic benefit in preventing inflammation-related tissue decline in later life. Video abstract.
Keywords: Aging; Fecal microbiota transplantation; Gut–brain axis; Gut–retina; Inflammaging; Intestine; Leaky gut; Microbiota.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Arbizu, PM. 2020. “PairwiseAdonis: pairwise multilevel comparison using Adonis. R Package Version 0.4.” https://github.com/pmartinezarbizu/pairwiseAdonis.
Publication types
MeSH terms
Grants and funding
- BB/CCG1860/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/N000250/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10353/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/F/000PR10355/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources

