Citalopram Did Not Significantly Improve Anxiety in Children with Autism Spectrum Disorder Undergoing Treatment for Core Symptoms: Secondary Analysis of a Trial to Reduce Repetitive Behaviors
- PMID: 35501967
- PMCID: PMC11075077
- DOI: 10.1089/cap.2021.0137
Citalopram Did Not Significantly Improve Anxiety in Children with Autism Spectrum Disorder Undergoing Treatment for Core Symptoms: Secondary Analysis of a Trial to Reduce Repetitive Behaviors
Abstract
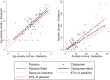
Objective: Anxiety disorders are among the most common co-occurring conditions in autism spectrum disorder (ASD). Despite their prevalence and impact, there are no randomized controlled trials (RCTs) aimed at evaluating the efficacy of selective serotonin reuptake inhibitors (SSRIs) for anxiolysis in this population, who may have a different biological basis for anxiety. Methods: Secondary analyses of the STAART double-blind, placebo-controlled RCT of citalopram in children with ASD examined whether citalopram reduced anxiety measured on the parent-reported Child and Adolescent Symptom Inventory-4 (CASI-4) as the primary outcome. An intention-to-treat analysis involving all 149 participants used multiple imputations for missing data and included baseline stratification factors of age group and site, among others. We prespecified as clinically significant a 33% reduction in anxiety in citalopram versus placebo, coinciding with 80% power. We tested whether communicative ability on the Vineland Communication score moderated treatment effect and explored whether initial anxiety was associated with greater adverse events, which could impact on dose titration and achieving optimal dose. Results: Both groups showed substantial reduction in anxiety. Citalopram was associated with a nonsignificant 16.5% greater reduction (observed coefficient = -0.181, bootstrap standard error = 0.126, p = 0.151, confidence interval = -0.428 to 0.066). Anxiety reports were significantly lower in children with reduced communicative ability, but communicative ability did not moderate the treatment effect (interaction p = 0.294). Initial anxiety levels were not associated with increased adverse effects (interaction ps 0.162-0.954). Conclusion: Citalopram did not statistically significantly improve anxiety in children with ASD. Clinicians should be cautious in their use of SSRIs for this indication. There remains a need for well-powered clinical trials testing the efficacy of SSRIs among autistic children with anxiety disorders.
Keywords: anxiety; autism; autistic disorder; randomized controlled trial; selective serotonin reuptake inhibitors.
Figures

References
-
- Aman MG, Lam KSL, Van Bourgondien ME: Medication patterns in patients with autism: Temporal, regional, and demographic influences. J Child Adolesc Psychopharmacol 15:116–126, 2005. - PubMed
-
- Aman MG, Singh NN: The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. Am J Ment Defic 89:485–491, 1985. - PubMed
-
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author.
-
- Baldwin D, Woods R, Lawson R, Taylor D: Efficacy of drug treatments for generalised anxiety disorder: Systematic review and meta-analysis. BMJ 342:d1199, 2011. - PubMed
-
- Buchsbaum MS, Hollander E, Haznedar MM, Tang C, Spiegel-Cohen J, Wei TC, Solimando A, Buchsbaum BR, Robins D, Bienstock C, Cartwright C, Mosovich S: Effect of fluoxetine on regional cerebral metabolism in autistic spectrum disorders: A pilot study. Int J Neuropsychopharmacol 4:119–125, 2001. - PubMed

