Topiramate-induced acute angle closure: A systematic review of case reports and case series
- PMID: 35502014
- PMCID: PMC9333044
- DOI: 10.4103/ijo.IJO_2134_21
Topiramate-induced acute angle closure: A systematic review of case reports and case series
Abstract
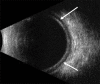
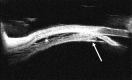
Topiramate-induced acute angle closure (TiAAC) is a potentially vision-threatening side effect of topiramate (TPM) use. The purpose of this article is to review demographic characteristics, clinical features, and management options of TiAAC. A systematic literature search of all reported cases and case series of TiAAC was conducted in the following search engines: PubMed, Web of Science, Google Scholar, Elsevier, and EBSCO. Seventy-three publications describing 77 cases were included. 58 (75.3%) patients were female, and the mean age was 34.88 ± 11.21 years (range, 7-57). The most commonly reported indication of TPM use was migraine headache (59.7%), and the mean duration from starting treatment until the onset of angle closure was 14.1 ± 31.5 days. All cases were managed by immediate cessation of TPM and topical therapy. In addition, systemic medications (carbonic anhydrase inhibitors, hyperosmotic agents, and steroids) were used in 51 patients (66.2%). A laser and/or surgical intervention was performed in 10 patients (13%). After commencement of treatment, the mean duration until the resolution of TiAAC was 3.9 ± 3.6 days (range, 1-18). The findings of our study present a summary of the current body of evidence provided by case reports and case series on TiAAC. In conclusion, the onset of angle closure following TPM use peaks at 2 weeks after initiating treatment, and in most cases, successful management can be achieved by discontinuing TPM and initiating appropriate medical therapy.
Keywords: Angle-closure; glaucoma; topiramate.
Conflict of interest statement
None
Figures
References
-
- Verrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S. Topiramate-induced weight loss: A review. Epilepsy Res. 2011;95:189–99. - PubMed
-
- Fritz N, Glogau S, Hoffmann J, Rademacher M, Elger CE, Helmstaedter C. Efficacy and cognitive side effects of tiagabine and topiramate in patients with epilepsy. Epilepsy Behav. 2005;6:373–81. - PubMed
-
- Mahmoud AAH, Rizk T, El-Bakri NK, Riaz M, Dannawi S, Al Tannir M. Incidence of kidney stones with topiramate treatment in pediatric patients. Epilepsia. 2011;52:1890–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

