Long-term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2-year results from two pivotal phase 3 studies
- PMID: 35502600
- PMCID: PMC9546219
- DOI: 10.1111/ejh.13783
Long-term safety and efficacy of ravulizumab in patients with paroxysmal nocturnal hemoglobinuria: 2-year results from two pivotal phase 3 studies
Abstract
Objectives: The complement component 5 (C5) inhibitor ravulizumab demonstrated non-inferiority to eculizumab following 26 weeks of treatment in complement inhibitor-naïve and complement inhibitor-experienced patients with paroxysmal nocturnal hemoglobinuria (PNH; studies 301 and 302, respectively). This study aims to describe the results of both studies from 27 weeks to 2 years.
Methods: Patients (N = 441) continued to receive ravulizumab throughout the extension period. Efficacy endpoints included lactate dehydrogenase (LDH) normalization, transfusion avoidance and fatigue score (FACIT-F). Safety analyses were also performed.
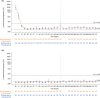
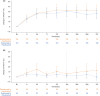
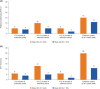
Results: From 27 weeks to 2 years, improvements in LDH levels were maintained in both study populations. Transfusion avoidance was maintained in 81.9% (study 301) and 85.6% (study 302) of patients, and FACIT-F scores remained stable. Ravulizumab was well tolerated, and the incidence of adverse events (AEs) were similar between patients of both studies. Incidence of serious AEs deemed related to ravulizumab treatment was low (<3%).
Conclusions: This study reports, to date, the longest period of follow-up in over 400 patients with PNH treated with ravulizumab (662 patient-years). Long-term, ravulizumab demonstrated durable efficacy and was well tolerated, highlighting the importance of C5 inhibitors as the mainstay of PNH treatment.
Keywords: breakthrough hemolysis; complement inhibitor; lactate dehydrogenase; paroxysmal nocturnal hemoglobinuria; ravulizumab.
© 2022 Alexion, AstraZeneca Rare Disease. European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
AGK has received consultancy fees/honoraria/speaker's bureau from Achillion, Akari, Alexion, AstraZeneca Rare Disease, Amgen, Apellis, Biocryst, Celgene, F. Hoffmann‐La Roche, Novartis and Ra Pharma and also research funding from Celgene. MG has received honoraria and advisory board from Alexion, AstraZeneca Rare Disease, and Sobi, consultancy fees and advisory board from BioCryst, consultancy fees from Regeneron Pharmaceuticals, and support for Medscape education in PNH from Apellis. KU has received research funding from Apellis, Alexion, AstraZeneca Rare Disease, Chugai and Roche, and speaker's bureau from Novartis. AK has received honoraria and research support (to Pavlov University) from Alexion, AstraZeneca Rare Disease. MO, JY and AM are employees and stockholders of Alexion, AstraZeneca Rare Disease. JN has received honoraria and provided consultancy to Alexion, AstraZeneca Rare Disease, Apellis, Biocryst, Chugai, Novartis and Roche. JWL received honoraria, consulting fees, and grants (to Seoul St. Mary's Hospital) from Alexion, AstraZeneca Rare Disease. RPL has provided consultancy, honoraria, membership on an Alexion, AstraZeneca Rare Disease. Board of Directors or advisory committees and has received research funding, Speaker's Bureau from Alexion, AstraZeneca Rare Disease.
Figures
References
-
- Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333:1253‐1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

