Microglia in brain development and regeneration
- PMID: 35502782
- PMCID: PMC9124570
- DOI: 10.1242/dev.200425
Microglia in brain development and regeneration
Abstract
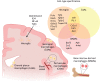
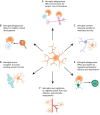
It has recently emerged that microglia, the tissue-resident macrophages of the central nervous system, play significant non-innate immune roles to support the development, maintenance, homeostasis and repair of the brain. Apart from being highly specialized brain phagocytes, microglia modulate the development and functions of neurons and glial cells through both direct and indirect interactions. Thus, recognizing the elements that influence the homeostasis and heterogeneity of microglia in normal brain development is crucial to understanding the mechanisms that lead to early disease pathogenesis of neurodevelopmental disorders. In this Review, we discuss recent studies that have elucidated the physiological development of microglia and summarize our knowledge of their non-innate immune functions in brain development and tissue repair.
Keywords: Brain development; Heterogeneity; Microglia; Neurons; Oligodendrocytes; Synapse.
© 2022. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

