Blockade of retinal or cortical activity does not prevent the development of callosal patches normally associated with ocular dominance columns in primary visual cortex
- PMID: 35502808
- PMCID: PMC8477274
- DOI: 10.1017/S0952523821000110
Blockade of retinal or cortical activity does not prevent the development of callosal patches normally associated with ocular dominance columns in primary visual cortex
Abstract
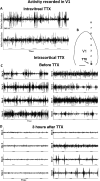
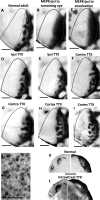
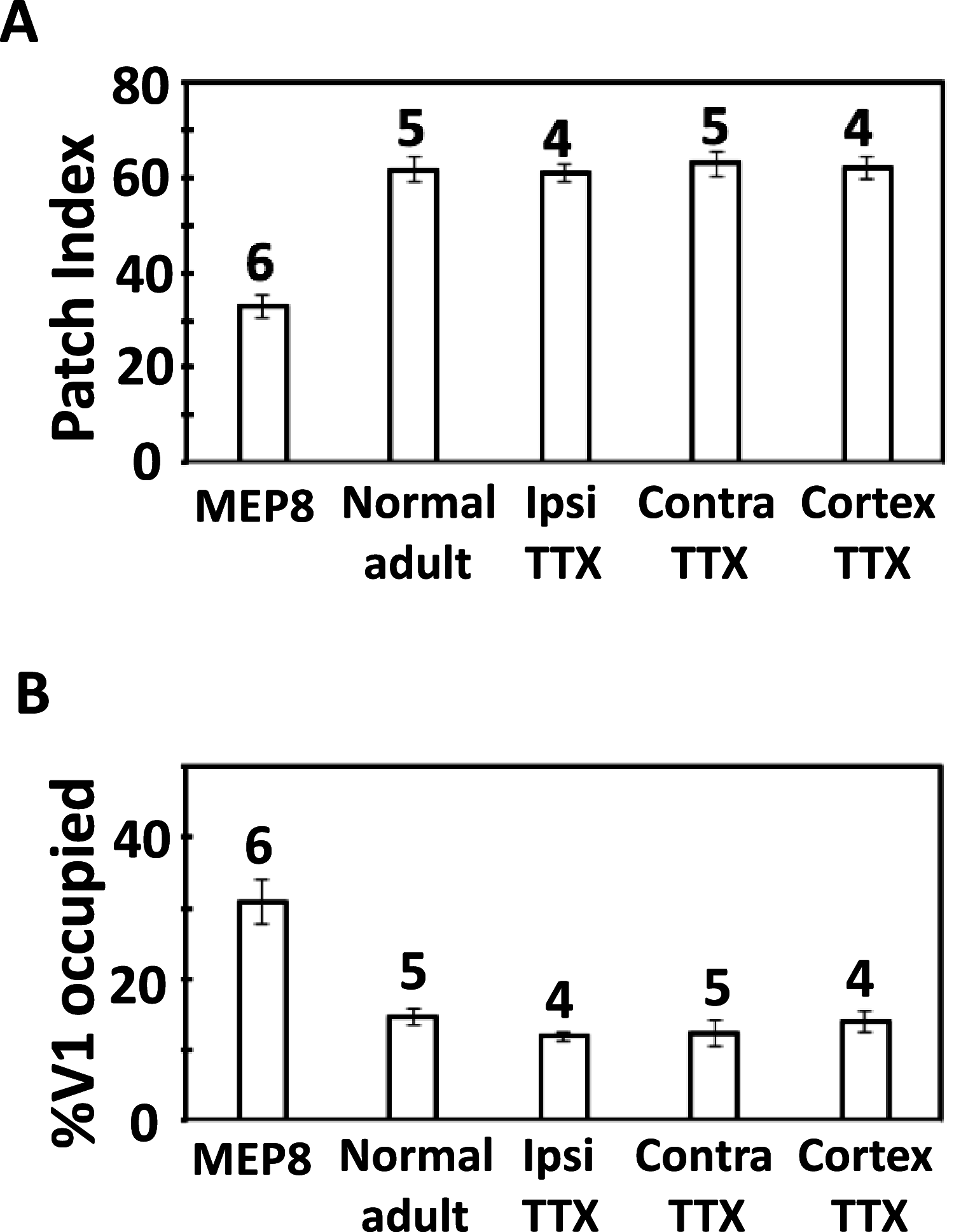
Callosal patches in primary visual cortex of Long Evans rats, normally associated with ocular dominance columns, emerge by postnatal day 10 (P10), but they do not form in rats monocularly enucleated a few days before P10. We investigated whether we could replicate the results of monocular enucleation by using tetrodotoxin (TTX) to block neural activity in one eye, or in primary visual cortex. Animals received daily intravitreal (P6-P9) or intracortical (P7-P9) injections of TTX, and our physiological evaluation of the efficacy of these injections indicated that the blockade induced by a single injection lasted at least 24 h. Four weeks later, the patterns of callosal connections in one hemisphere were revealed after multiple injections of horseradish peroxidase in the other hemisphere. We found that in rats receiving either intravitreal or cortical injections of TTX, the patterns of callosal patches analyzed in tangential sections from the flattened cortex were not significantly different from the pattern in normal rats. Our findings, therefore, suggest that the effects of monocular enucleation on the distribution of callosal connections are not due to the resulting imbalance of afferent ganglion cell activity, and that factors other than neural activity are likely involved.
Keywords: Long Evans rats; columnar organization; interhemispheric connections; segregation; tetrodotoxin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Ocular dominance columns in V1 are more susceptible than associated callosal patches to imbalance of eye input during precritical and critical periods.J Comp Neurol. 2021 Aug 1;529(11):2883-2910. doi: 10.1002/cne.25134. Epub 2021 Mar 17. J Comp Neurol. 2021. PMID: 33683706 Free PMC article.
-
Callosal contribution to ocular dominance in rat primary visual cortex.Eur J Neurosci. 2010 Oct;32(7):1163-9. doi: 10.1111/j.1460-9568.2010.07363.x. Epub 2010 Aug 19. Eur J Neurosci. 2010. PMID: 20726891
-
The callosal pattern in striate cortex is more patchy in monocularly enucleated albino than pigmented rats.Neurosci Lett. 1996 Feb 9;204(3):169-72. doi: 10.1016/0304-3940(96)12359-4. Neurosci Lett. 1996. PMID: 8938257
-
Effect of monocular blockade of retinal activity on the development of visual callosal connections in the rat.Biol Res. 1995;28(3):219-26. Biol Res. 1995. PMID: 9251752
-
Cellular aspects of callosal connections and their development.Neuropsychologia. 1995 Aug;33(8):961-87. doi: 10.1016/0028-3932(95)00033-y. Neuropsychologia. 1995. PMID: 8524456 Review.
Cited by
-
A column-like organization for ocular dominance in mouse visual cortex.Nat Commun. 2025 Feb 25;16(1):1926. doi: 10.1038/s41467-025-56780-3. Nat Commun. 2025. PMID: 40000624 Free PMC article.
References
-
- Chang, K., Van Sluyters, R.C., & Olavarria, J. (1995). Effect of monocular blockade of retinal activity on the development of visual callosal connections in the rat. Biological Research 28, 219–226. - PubMed
-
- Cook, P.M., Prusky, G. & Ramoa, A.S. (1999). The role of spontaneous retinal activity before eye opening in the maturation of form and function in the retinogeniculate pathway of the ferret. Visual Neuroscience 16, 491–501. - PubMed
-
- Cusick, C.G. & Lund, R.D. (1982). Modification of visual callosal projections in rats. The Journal of Comparative Neurology 212, 385–398. - PubMed
-
- Fagiolini, M., Pizzorusso, T., Berardi, N., Domeneci, L., & Maffei, L. (1994). Functional postnatal development of the rat primary visual cortex and the role of visual experience: Dark rearing and monocular deprivation. Visual Research 34, 709–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

