GTM-3, an Extra-Large Pore Enantioselective Chiral Zeolitic Catalyst
- PMID: 35502872
- PMCID: PMC9100664
- DOI: 10.1021/jacs.2c01874
GTM-3, an Extra-Large Pore Enantioselective Chiral Zeolitic Catalyst
Abstract
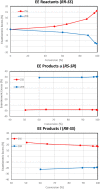
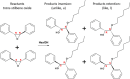
The development of chiral zeolitic catalysts possessing extra-large pores and endowed with the capability of enantioselectively processing bulky products represents one of the greatest challenges in chemistry. Here, we report the discovery of GTM-3, an enantio-enriched extra-large pore chiral zeolite material with -ITV framework structure, obtained using a simple enantiopure organic cation derived from the chiral pool, N,N-ethyl-methyl-pseudoephedrinium, as the chiral-inductor agent. We demonstrate the enantio-enrichment of GTM-3 in one of the two enantiomorphic polymorphs using the two enantiomers of the organic cation. Interestingly, we prove the ability of this zeolitic material to perform enantioselective catalytic operations with very large substrates, here exemplified by the catalytic epoxide aperture of the bulky trans-stilbene oxide with alcohols, yielding unprecedented product enantiomeric excesses up to 30%. Our discovery opens the way for the use of accessible chiral zeolitic materials for the catalytic asymmetric synthesis of chiral pharmaceutical compounds.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Lough W. J.; Wainer I. W.. Chirality in Natural and Applied Science, Blackwell, 2002; ISBN 9780849324345.
-
- Rouhi M. Top Pharmaceuticals: Thalidomide. Chem. Eng. News 2005, 83, 122–2005. 10.1021/cen-v083n025.p122. - DOI
-
- Harris K. D. M.; Thomas S. J. M. Selected thoughts on chiral crystals, chiral surfaces, and asymmetric heterogeneous catalysis. ChemCatChem. 2009, 1, 223–231. 10.1002/cctc.200900181. - DOI
-
- Davis M. E. Reflections on routes to enantioselective solid catalysts. Top. Catal. 2003, 25, 3–7. 10.1023/B:TOCA.0000003093.74240.23. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

