Proteolysis of fibrillin-2 microfibrils is essential for normal skeletal development
- PMID: 35503090
- PMCID: PMC9064305
- DOI: 10.7554/eLife.71142
Proteolysis of fibrillin-2 microfibrils is essential for normal skeletal development
Abstract
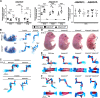
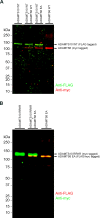
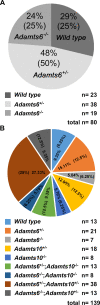
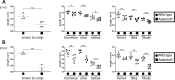
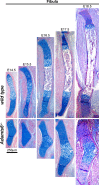
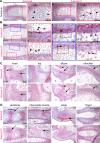
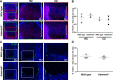
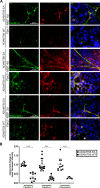
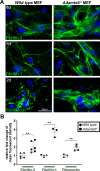
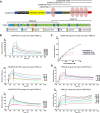
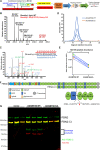
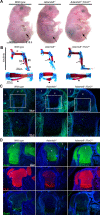
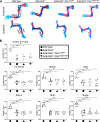
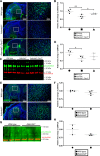
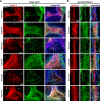
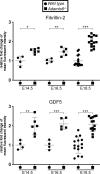
The embryonic extracellular matrix (ECM) undergoes transition to mature ECM as development progresses, yet few mechanisms ensuring ECM proteostasis during this period are known. Fibrillin microfibrils are macromolecular ECM complexes serving structural and regulatory roles. In mice, Fbn1 and Fbn2, encoding the major microfibrillar components, are strongly expressed during embryogenesis, but fibrillin-1 is the major component observed in adult tissue microfibrils. Here, analysis of Adamts6 and Adamts10 mutant mouse embryos, lacking these homologous secreted metalloproteases individually and in combination, along with in vitro analysis of microfibrils, measurement of ADAMTS6-fibrillin affinities and N-terminomics discovery of ADAMTS6-cleaved sites, identifies a proteostatic mechanism contributing to postnatal fibrillin-2 reduction and fibrillin-1 dominance. The lack of ADAMTS6, alone and in combination with ADAMTS10 led to excess fibrillin-2 in perichondrium, with impaired skeletal development defined by a drastic reduction of aggrecan and cartilage link protein, impaired BMP signaling in cartilage, and increased GDF5 sequestration in fibrillin-2-rich tissue. Although ADAMTS6 cleaves fibrillin-1 and fibrillin-2 as well as fibronectin, which provides the initial scaffold for microfibril assembly, primacy of the protease-substrate relationship between ADAMTS6 and fibrillin-2 was unequivocally established by reversal of the defects in Adamts6-/- embryos by genetic reduction of Fbn2, but not Fbn1.
Keywords: ADAMTS; Protease; developmental biology; extracellular matrix; fibrillin; mouse; proteolysis; skeletal development.
© 2022, Mead et al.
Conflict of interest statement
TM, DM, LW, SC, CG, EC, JM, DR, CL, CB, SA No competing interests declared
Figures




















References
-
- Bader HL, Ruhe AL, Wang LW, Wong AK, Walsh KF, Packer RA, Mitelman J, Robertson KR, O’Brien DP, Broman KW, Shelton GD, Apte SS, Neff MW. An ADAMTSL2 founder mutation causes Musladin-Lueke Syndrome, a heritable disorder of beagle dogs, featuring stiff skin and joint contractures. PLOS ONE. 2010;5:e12817. doi: 10.1371/journal.pone.0012817. - DOI - PMC - PubMed
-
- Bader HL, Wang LW, Ho JC, Tran T, Holden P, Fitzgerald J, Atit RP, Reinhardt DP, Apte SS. A disintegrin-like and metalloprotease domain containing thrombospondin type 1 motif-like 5 (ADAMTSL5) is A novel fibrillin-1-, fibrillin-2-, and heparin-binding member of the ADAMTS superfamily containing A netrin-like module. Matrix Biology. 2012;31:398–411. doi: 10.1016/j.matbio.2012.09.003. - DOI - PMC - PubMed
-
- Beene LC, Wang LW, Hubmacher D, Keene DR, Reinhardt DP, Annis DS, Mosher DF, Mecham RP, Traboulsi EI, Apte SS. Nonselective assembly of fibrillin 1 and fibrillin 2 in the rodent ocular zonule and in cultured cells: implications for Marfan syndrome. Investigative Ophthalmology & Visual Science. 2013;54:8337–8344. doi: 10.1167/iovs.13-13121. - DOI - PMC - PubMed
-
- Bekhouche M, Leduc C, Dupont L, Janssen L, Delolme F, Vadon-Le Goff S, Smargiasso N, Baiwir D, Mazzucchelli G, Zanella-Cleon I, Dubail J, De Pauw E, Nusgens B, Hulmes DJ, Moali C, Colige A. Determination of the substrate repertoire of ADAMTS2, 3, and 14 significantly broadens their functions and identifies extracellular matrix organization and TGF-beta signaling as primary targets. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2016;30:1741–1756. doi: 10.1096/fj.15-279869. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

