Sustaining Antimicrobial Stewardship in a High-Antibiotic Resistance Setting
- PMID: 35503216
- PMCID: PMC9066280
- DOI: 10.1001/jamanetworkopen.2022.10180
Sustaining Antimicrobial Stewardship in a High-Antibiotic Resistance Setting
Abstract
Importance: There is a lack of studies comparing the intended and unintended consequences of prospective review and feedback (PRF) with computerized decision support systems (CDSS), especially in the longer term in antimicrobial stewardship.
Objective: To examine the outcomes associated with the sequential implementation of PRF and CDSS and changes to these interventions with long-term use of antibiotics for and incidence of multidrug resistant organisms (MDROs) and other unintended outcomes.
Design, setting, and participants: This cohort study used an interrupted time series with segmented regression analysis of data from January 2007 to December 2018. Data were extracted from the electronic medical records of patients admitted at a large university teaching hospital with high rates of antibiotic resistance in Singapore. Data were analyzed from June 2019 to June 2020.
Exposures: PRF of piperacillin-tazobactam and carbapenems (intervention 1, April 2009), with the addition of hospital-wide CDSS (intervention 2, April 2011), and lifting of CDSS for half of the hospital wards for 6 months (intervention 3, March 2017).
Main outcomes and measures: Monthly antimicrobial use was measured in defined daily doses (DDDs) per 1000 patient-days. The monthly incidence of MDROs was calculated as number of clinical isolates detected per 1000 inpatient-days over a 6-month period. Unintended outcomes examined included in-hospital mortality and age-adjusted length of stay (LOS).
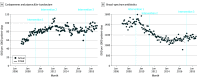
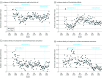
Results: The number of inpatients increased from 56 263 in 2007 to 63 572 in 2018. During the same period, the mean monthly patient days increased from 33 929 in 2007 to 45 603 in 2018, and the proportion of patients older than 65 years increased from 45.5% in 2007 to 56.6% in 2018. After intervention 1, there were 0.33 (95% CI, 0.18 to 0.48) more DDDs per 1000 patient-days per month of piperacillin-tazobactam and carbapenems and -11.05 (95% CI, -15.55 to -6.55) fewer DDDs per 1000 patient-days per month for other broad-spectrum antibiotics. After intervention 2, there were -0.22 (95% CI, -0.33 to -0.10) fewer DDDs per 1000 patient-days per month of piperacillin-tazobactam and carbapenems and -2.10 (95% CI, -3.13 to -1.07) fewer DDDs per 1000 patient-days per month for other broad-spectrum antibiotics. After intervention 3, use of piperacillin-tazobactam and carbapenem increased by 0.28 (95% CI, 0.02 to 0.55) DDDs per 1000 patient-days per month. After intervention 2, incidence of Clostridioides difficile decreased (estimate, -0.02 [95% CI, -0.03 to -0.01] cases per 1000 patient-days per month).
Conclusions and relevance: In this cohort study, concurrent PRF and CDSS were associated with limiting the use of piperacillin-tazobactam and carbapenems while reducing use of other antibiotics.
Conflict of interest statement
Figures

References
-
- World Health Organization . The selection and use of essential medicines: 2017. Accessed October 29, 2021. https://www.who.int/medicines/publications/essentialmedicines/EML_2017_E...
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

