Cathepsin B Relocalization in Late Membrane Disrupted Neurons Following Diffuse Brain Injury in Rats
- PMID: 35503242
- PMCID: PMC9069603
- DOI: 10.1177/17590914221099112
Cathepsin B Relocalization in Late Membrane Disrupted Neurons Following Diffuse Brain Injury in Rats
Abstract
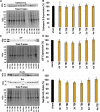
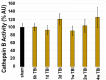
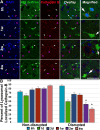
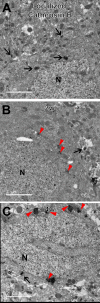
Traumatic brain injury (TBI) has consequences that last for years following injury. While TBI can precipitate a variety of diffuse pathologies, the mechanisms involved in injury-induced neuronal membrane disruption remain elusive. The lysosomal cysteine protease, Cathepsin B (Cath B), and specifically its redistribution into the cytosol has been implicated in cell death. Little is known about Cath B or neuronal membrane disruption chronically following diffuse TBI. Therefore, the current study evaluated Cath B and diffuse neuronal membrane disruption over a more chronic post-injury window (6 h-4 w). We evaluated Cath B in adult male Sprague-Dawley rats following central fluid percussion injury (CFPI). Expression of Cath B, as well as Cath B-associated pro (Bak and AIF) and anti-apoptotic (Bcl-xl) proteins, were assessed using western blot analysis. Cath B activity was also assessed. Localization of Cath B was evaluated in the membrane disrupted and non-disrupted population following CFPI using immunohistochemistry paired with quantitative image analysis and ultrastructural verification. There was no difference in expression or activity of Cath B or any of the associated proteins between sham and CFPI at any time post-injury. Immunohistological studies, however, showed a sub-cellular re-localization of Cath B at 2 w and 4 w post-injury in the membrane disrupted neuronal population as compared to the time-point matched non-disrupted neurons. Both membrane disruption and Cath B relocalization appear linked to neuronal atrophy. These observations are indicative of a late secondary pathology that represents an opportunity for therapeutic treatment of these neurons following diffuse TBI. Summary Statement Lysosomal cathepsin B relocalizes to the cytosol in neurons with disrupted plasmalemmal membranes weeks following diffuse brain injury. Both the membrane disrupted and cathepsin B relocalized neuronal subpopulations displayed smaller soma and nucleus size compared to non-pathological neurons, indicating atrophy.
Keywords: AIF; Bak/Bcl-XL; cathepsin B; membrane disruption; neuronal atrophy; traumatic brain injury.
Conflict of interest statement
Figures
References
-
- Amritraj A., Peake K., Kodam A., Salio C., Merighi A., Vance J. E., Kar S. (2009). Increased activity and altered subcellular distribution of lysosomal enzymes determine neuronal vulnerability in Niemann-Pick type C1-deficient mice. The American Journal of Pathology, 175(6), 2540–2556. 10.2353/ajpath.2009.081096 - DOI - PMC - PubMed
-
- Boutté A. M., Hook V., Thangavelu B., Sarkis G. A., Abbatiello B. N., Hook G., Jacobsen J. S., Gilsdorf J, Yang Z., Wang K. K. W., Shear D. A. (2020). Penetrating traumatic brain injury triggers dysregulation of Cathepsin B protein levels independent of cysteine protease activity in brain and cerebral spinal fluid. Journal of Neurotrauma, 37(13), 1574–1586. 10.1089/neu.2019.6537 - DOI - PMC - PubMed
-
- Boya P., Andreau K., Poncet D., Zamzami N., Perfettini J. L., Metivier D., Ojcius D. M., Jäättelä M., Kroemer G. (2003). Lysosomal membrane permeabilization induces cell death in a mitochondrion-dependent fashion. Journal of Experimental Medicine, 197(10), 1323–1334. 10.1084/jem.20021952 - DOI - PMC - PubMed
-
- Cavallo-Medved D., Moin K., Sloane B. (2011). Cathepsin B: basis sequence: mouse. AFCS-nature Molecule Pages, 2011, 1–17. PMCID: PMC5541861 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

