IL-33 acts as a costimulatory signal to generate alloreactive Th1 cells in graft-versus-host disease
- PMID: 35503257
- PMCID: PMC9197517
- DOI: 10.1172/JCI150927
IL-33 acts as a costimulatory signal to generate alloreactive Th1 cells in graft-versus-host disease
Abstract
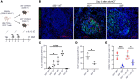
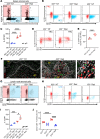
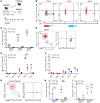
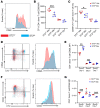
Antigen-presenting cells (APCs) integrate signals emanating from local pathology and program appropriate T cell responses. In allogeneic hematopoietic stem cell transplantation (alloHCT), recipient conditioning releases damage-associated molecular patterns (DAMPs) that generate proinflammatory APCs that secrete IL-12, which is a driver of donor Th1 responses, causing graft-versus-host disease (GVHD). Nevertheless, other mechanisms exist to initiate alloreactive T cell responses, as recipients with disrupted DAMP signaling or lacking IL-12 develop GVHD. We established that tissue damage signals are perceived directly by donor CD4+ T cells and promoted T cell expansion and differentiation. Specifically, the fibroblastic reticular cell-derived DAMP IL-33 is increased by recipient conditioning and is critical for the initial activation, proliferation, and differentiation of alloreactive Th1 cells. IL-33 stimulation of CD4+ T cells was not required for lymphopenia-induced expansion, however. IL-33 promoted IL-12-independent expression of Tbet and generation of Th1 cells that infiltrated GVHD target tissues. Mechanistically, IL-33 augmented CD4+ T cell TCR-associated signaling pathways in response to alloantigen. This enhanced T cell expansion and Th1 polarization, but inhibited the expression of regulatory molecules such as IL-10 and Foxp3. These data establish an unappreciated role for IL-33 as a costimulatory signal for donor Th1 generation after alloHCT.
Keywords: Bone marrow transplantation; Costimulation; Immunology; Th1 response; Transplantation.
Conflict of interest statement
Figures



Comment in
-
Graft-versus-host disease: establishing IL-33 as an important costimulatory molecule.J Clin Invest. 2022 Jun 15;132(12):e160692. doi: 10.1172/JCI160692. J Clin Invest. 2022. PMID: 35703182 Free PMC article.
Similar articles
-
Graft-versus-host disease: establishing IL-33 as an important costimulatory molecule.J Clin Invest. 2022 Jun 15;132(12):e160692. doi: 10.1172/JCI160692. J Clin Invest. 2022. PMID: 35703182 Free PMC article.
-
Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease.Blood. 2008 Sep 1;112(5):2101-10. doi: 10.1182/blood-2007-12-126987. Epub 2008 Jul 2. Blood. 2008. PMID: 18596226 Free PMC article.
-
Graft-versus-host disease is independent of innate signaling pathways triggered by pathogens in host hematopoietic cells.J Immunol. 2011 Jan 1;186(1):230-41. doi: 10.4049/jimmunol.1002965. Epub 2010 Nov 22. J Immunol. 2011. PMID: 21098219 Free PMC article.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
The primacy of gastrointestinal tract antigen-presenting cells in lethal graft-versus-host disease.Blood. 2019 Dec 12;134(24):2139-2148. doi: 10.1182/blood.2019000823. Blood. 2019. PMID: 31697827 Free PMC article. Review.
Cited by
-
Dual Immune Regulatory Roles of Interleukin-33 in Pathological Conditions.Cells. 2022 Oct 14;11(20):3237. doi: 10.3390/cells11203237. Cells. 2022. PMID: 36291105 Free PMC article. Review.
-
T lymphocyte-dependent IL-10 down-regulates a cytokine storm driven by Toxoplasma gondii GRA24.mBio. 2024 Nov 13;15(11):e0145524. doi: 10.1128/mbio.01455-24. Epub 2024 Oct 23. mBio. 2024. PMID: 39440975 Free PMC article.
-
The graft versus leukemia effect: donor lymphocyte infusions and cellular therapy.Front Immunol. 2024 Mar 15;15:1328858. doi: 10.3389/fimmu.2024.1328858. eCollection 2024. Front Immunol. 2024. PMID: 38558819 Free PMC article. Review.
-
Type II innate lymphoid cell plasticity contributes to impaired reconstitution after allogeneic hematopoietic stem cell transplantation.Nat Commun. 2024 Jul 17;15(1):6000. doi: 10.1038/s41467-024-50263-7. Nat Commun. 2024. PMID: 39019846 Free PMC article.
-
Extracellular release of damaged mitochondria induced by prehematopoietic stem cell transplant conditioning exacerbates GVHD.Blood Adv. 2024 Jul 23;8(14):3691-3704. doi: 10.1182/bloodadvances.2023012328. Blood Adv. 2024. PMID: 38701354 Free PMC article.
References
-
- Klein OR, et al. Nonmyeloablative haploidentical bone marrow transplantation with post-transplantation cyclophosphamide for pediatric and young adult patients with high-risk hematologic malignancies. Biol Blood Marrow Transplant. 2017;23(2):325–332. doi: 10.1016/j.bbmt.2016.11.016. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

