Quantitative autofluorescence findings in patients undergoing hydroxychloroquine treatment
- PMID: 35503294
- PMCID: PMC9545387
- DOI: 10.1111/ceo.14090
Quantitative autofluorescence findings in patients undergoing hydroxychloroquine treatment
Abstract
Background: To measure quantitative autofluorescence (qAF) in patients under treatment with hydroxychloroquine (HCQ) and at risk of retinal toxicity but with no apparent signs of retinal toxicity and to compare it with that of untreated subjects.
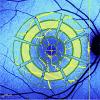
Methods: Consecutive patients at risk for the development of HCQ retinal toxicity (duration of treatment >5 years or daily HCQ dose >5 mg/kg of actual body weight [ABW]) but no alterations on spectral domain-optical coherence tomography, short-wavelength autofluorescence and 10-2 visual field examination were recruited. Healthy subjects matched by age and sex were also enrolled in the study. All subjects underwent qAF measurements in one eye. Images were analysed using the conventional qAF grid by Delori calculating the qAF of eight sectors of the intermediate ring and the mean of those values (qAF8 ).
Results: Thirty-nine patients treated with HCQ (38 females, mean age 52.1 ± 8.6 years) and 39 untreated subjects (38 females, mean age 51.2 ± 8.6 years) were included. In both HCQ patients and untreated subjects, qAF8 was positively correlated with age (p = 0.004). Although HCQ patients showed a higher mean qAF8 compared with untreated subjects (294.7 ± 65.3 vs. 268.9 ± 57.5), the difference was not significant (p = 0.068). HCQ patients showed significantly higher mean qAF values in the inferior-temporal, inferior and inferior-nasal sectors of the intermediate ring of qAF grid compared with untreated subjects (all p < 0.05).
Conclusions: These results suggest a possible preclinical increase of qAF values in inferior parafoveal sectors probably induced by HCQ exposure.
Keywords: hydroxychloroquine; hydroxychloroquine retinopathy screening; multimodal imaging; quantitative autofluorescence.
© 2022 The Authors. Clinical & Experimental Ophthalmology published by John Wiley & Sons Australia, Ltd on behalf of Royal Australian and New Zealand College of Ophthalmologists.
Conflict of interest statement
Salvatore Parrulli, no financial disclosures; Mariano Cozzi, Recipient: Bayer, Nidek, Zeiss; Matteo Airaldi, no financial disclosures; Francesco Romano, no financial disclosures; Francesco Viola, Novartis (C), Bayer (C), Roche (C); Piercarlo Sarzi‐Puttini, no financial disclosures; Giovanni Staurenghi, Heidelberg Engineering (C), QuantelMedical (C), Centervue (C), Carl Zeiss Meditec (C), Alcon (C), Allergan (C), Bayer (C), Boheringer (C), Genentech (C), GSK (C), Novartis (C), and Roche (C), Optos (F), Optovue (F) and Centervue (F); Alessandro Invernizzi, Novartis (C), Bayer (C).
Figures



References
-
- Fernandez AP. Updated recommendations on the use of hydroxychloroquine in dermatologic practice. J Am Acad Dermatol. 2017;76(6):1176‐1182. - PubMed
-
- Rosenthal AR, Kolb H, Bergsma D, Huxsoll D, Hopkins JL. Chloroquine retinopathy in the rhesus monkey. Invest Ophthalmol Vis Sci. 1978;17(12):1158‐1175. - PubMed

