Neonatal injury models: integral tools to decipher the molecular basis of cardiac regeneration
- PMID: 35503383
- PMCID: PMC9064850
- DOI: 10.1007/s00395-022-00931-w
Neonatal injury models: integral tools to decipher the molecular basis of cardiac regeneration
Abstract
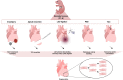
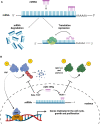
Myocardial injury often leads to heart failure due to the loss and insufficient regeneration of resident cardiomyocytes. The low regenerative potential of the mammalian heart is one of the main drivers of heart failure progression, especially after myocardial infarction accompanied by large contractile muscle loss. Preclinical therapies for cardiac regeneration are promising, but clinically still missing. Mammalian models represent an excellent translational in vivo platform to test drugs and treatments for the promotion of cardiac regeneration. Particularly, short-lived mice offer the possibility to monitor the outcome of such treatments throughout the life span. Importantly, there is a short period of time in newborn mice in which the heart retains full regenerative capacity after cardiac injury, which potentially also holds true for the neonatal human heart. Thus, in vivo neonatal mouse models of cardiac injury are crucial to gain insights into the molecular mechanisms underlying the cardiac regenerative processes and to devise novel therapeutic strategies for the treatment of diseased adult hearts. Here, we provide an overview of the established injury models to study cardiac regeneration. We summarize pioneering studies that demonstrate the potential of using neonatal cardiac injury models to identify factors that may stimulate heart regeneration by inducing endogenous cardiomyocyte proliferation in the adult heart. To conclude, we briefly summarize studies in large animal models and the insights gained in humans, which may pave the way toward the development of novel approaches in regenerative medicine.
Keywords: Cardiac regeneration; Cardiomyocyte proliferation; Myocardial infarction; Neonatal heart injury; Regenerative medicine; microRNA.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interest in relation to this work.
Figures

References
-
- Andersen DC, Jensen CH, Baun C, Hvidsten S, Zebrowski DC, Engel FB, Sheikh SP. Persistent scarring and dilated cardiomyopathy suggest incomplete regeneration of the apex resected neonatal mouse myocardium—a 180 days follow up study. J Mol Cell Cardiol. 2016;90:47–52. doi: 10.1016/j.yjmcc.2015.11.031. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

