Differential Modulation of Nuclear Receptor LRH-1 through Targeting Buried and Surface Regions of the Binding Pocket
- PMID: 35503419
- PMCID: PMC10026694
- DOI: 10.1021/acs.jmedchem.2c00235
Differential Modulation of Nuclear Receptor LRH-1 through Targeting Buried and Surface Regions of the Binding Pocket
Abstract
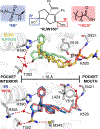
Liver receptor homologue-1 (LRH-1) is a phospholipid-sensing nuclear receptor that has shown promise as a target for alleviating intestinal inflammation and metabolic dysregulation in the liver. LRH-1 contains a large ligand-binding pocket, but generating synthetic modulators has been challenging. We have had recent success generating potent and efficacious agonists through two distinct strategies. We targeted residues deep within the pocket to enhance compound binding and residues at the mouth of the pocket to mimic interactions made by phospholipids. Here, we unite these two designs into one molecule to synthesize the most potent LRH-1 agonist to date. Through a combination of global transcriptomic, biochemical, and structural studies, we show that selective modulation can be driven through contacting deep versus surface polar regions in the pocket. While deep pocket contacts convey high affinity, contacts with the pocket mouth dominate allostery and provide a phospholipid-like transcriptional response in cultured cells.
Conflict of interest statement
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
Figures





Similar articles
-
A phospholipid mimetic targeting LRH-1 ameliorates colitis.Cell Chem Biol. 2022 Jul 21;29(7):1174-1186.e7. doi: 10.1016/j.chembiol.2022.03.001. Epub 2022 Mar 21. Cell Chem Biol. 2022. PMID: 35316658 Free PMC article.
-
Crystal Structures of the Nuclear Receptor, Liver Receptor Homolog 1, Bound to Synthetic Agonists.J Biol Chem. 2016 Dec 2;291(49):25281-25291. doi: 10.1074/jbc.M116.753541. Epub 2016 Sep 30. J Biol Chem. 2016. PMID: 27694446 Free PMC article.
-
Comparison of activity, structure, and dynamics of SF-1 and LRH-1 complexed with small molecule modulators.J Biol Chem. 2023 Aug;299(8):104921. doi: 10.1016/j.jbc.2023.104921. Epub 2023 Jun 14. J Biol Chem. 2023. PMID: 37328104 Free PMC article.
-
Molecular basis for the regulation of the nuclear receptor LRH-1.Curr Opin Cell Biol. 2015 Apr;33:26-34. doi: 10.1016/j.ceb.2014.10.007. Epub 2014 Nov 15. Curr Opin Cell Biol. 2015. PMID: 25463843 Review.
-
Are those phospholipids in your pocket?Cell Metab. 2005 Mar;1(3):153-5. doi: 10.1016/j.cmet.2005.02.006. Cell Metab. 2005. PMID: 16054056 Review.
Cited by
-
Illuminating ligand-induced dynamics in nuclear receptors through MD simulations.Biochim Biophys Acta Gene Regul Mech. 2024 Jun;1867(2):195025. doi: 10.1016/j.bbagrm.2024.195025. Epub 2024 Apr 16. Biochim Biophys Acta Gene Regul Mech. 2024. PMID: 38614450 Free PMC article. Review.
-
A novel heuristic of rigid docking scores positively correlates with full-length nuclear receptor LRH-1 regulation.Comput Struct Biotechnol J. 2024 Jul 30;23:3065-3080. doi: 10.1016/j.csbj.2024.07.021. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39185441 Free PMC article.
-
Liver receptor homolog-1: structures, related diseases, and drug discovery.Acta Pharmacol Sin. 2024 Aug;45(8):1571-1581. doi: 10.1038/s41401-024-01276-x. Epub 2024 Apr 17. Acta Pharmacol Sin. 2024. PMID: 38632319 Free PMC article. Review.
-
Enhanced dynamic coupling in a nuclear receptor underlies ligand activity.J Biol Chem. 2025 Feb;301(2):108081. doi: 10.1016/j.jbc.2024.108081. Epub 2024 Dec 14. J Biol Chem. 2025. PMID: 39675705 Free PMC article.
-
LRH-1 induces hepatoprotective nonessential amino acids in response to acute liver injury.J Clin Invest. 2023 Apr 3;133(7):e168805. doi: 10.1172/JCI168805. J Clin Invest. 2023. PMID: 37009899 Free PMC article.
References
-
- Nakajima T; Uchida U; Anderson SF; Lee C; Hurwitz J; Parvin JD; Montminy M Rna Helicase a Mediates Association of Cbp with Rna Polymerase II. Cell 1997, 90, 1107–1112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

