Bitter taste signaling in tracheal epithelial brush cells elicits innate immune responses to bacterial infection
- PMID: 35503420
- PMCID: PMC9246383
- DOI: 10.1172/JCI150951
Bitter taste signaling in tracheal epithelial brush cells elicits innate immune responses to bacterial infection
Abstract
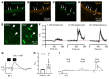
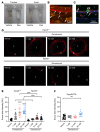
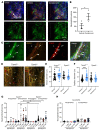
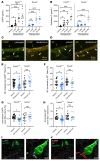
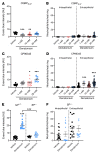
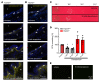
Constant exposure of the airways to inhaled pathogens requires efficient early immune responses protecting against infections. How bacteria on the epithelial surface are detected and first-line protective mechanisms are initiated are not well understood. We have recently shown that tracheal brush cells (BCs) express functional taste receptors. Here we report that bitter taste signaling in murine BCs induces neurogenic inflammation. We demonstrate that BC signaling stimulates adjacent sensory nerve endings in the trachea to release the neuropeptides CGRP and substance P that mediate plasma extravasation, neutrophil recruitment, and diapedesis. Moreover, we show that bitter tasting quorum-sensing molecules from Pseudomonas aeruginosa activate tracheal BCs. BC signaling depends on the key taste transduction gene Trpm5, triggers secretion of immune mediators, among them the most abundant member of the complement system, and is needed to combat P. aeruginosa infections. Our data provide functional insight into first-line defense mechanisms against bacterial infections of the lung.
Keywords: Immunology; Innate immunity; Pulmonology.
Figures




Comment in
-
Brush cells fine-tune neurogenic inflammation in the airways.J Clin Invest. 2022 Jul 1;132(13):e161439. doi: 10.1172/JCI161439. J Clin Invest. 2022. PMID: 35775485 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

