Corticotropin releasing factor (CRF) systems: Promoting cocaine pursuit without distress via incentive motivation
- PMID: 35503756
- PMCID: PMC9064096
- DOI: 10.1371/journal.pone.0267345
Corticotropin releasing factor (CRF) systems: Promoting cocaine pursuit without distress via incentive motivation
Abstract
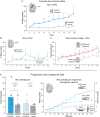
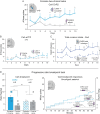
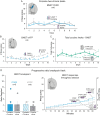
Corticotropin releasing factor (CRF) systems in limbic structures are posited to mediate stress-induced relapse in addiction, traditionally by generating distress states that spur drug consumption as attempts at hedonic self-medication. Yet evidence suggests that activating CRF-expressing neurons in the central amygdala (CeA) or nucleus accumbens (NAc) can magnify incentive motivation in absence of distress, at least for sucrose rewards. However, traditional CRF hypotheses in addiction neuroscience are primarily directed toward drug rewards. The question remains open whether CRF systems can similarly act via incentive motivation mechanisms to promote pursuit of drug rewards, such as cocaine. Here we tested whether optogenetic excitation of CRF-containing neurons in either NAc medial shell, lateral CeA, or dorsolateral BNST of transgenic Crh-Cre+ rats would spur preference and pursuit of a particular laser-paired cocaine reward over an alternative cocaine reward, and whether excitation served as a positively-valenced incentive itself, through laser self-stimulation tests. We report that excitation of CRF-containing neurons in either NAc or CeA recruited mesocorticolimbic circuitry to amplify incentive motivation to pursue the laser-paired cocaine: focusing preference on the laser-paired cocaine reward in a two-choice task, and spurred pursuit as doubled breakpoint in a progressive ratio task. Crucially indicating positive-valence, excitation of CRF neurons in NAc and CeA also was actively sought after by most rats in self-stimulation tasks. Conversely, CRF neuronal activation in BNST was never self-stimulated, but failed to enhance cocaine consumption. Collectively, we find that NAc and CeA CRF-containing neurons can amplify pursuit and consumption of cocaine by positively-valenced incentive mechanisms, without any aversive distress.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
Activating Corticotropin-Releasing Factor Systems in the Nucleus Accumbens, Amygdala, and Bed Nucleus of Stria Terminalis: Incentive Motivation or Aversive Motivation?Biol Psychiatry. 2021 Jun 15;89(12):1162-1175. doi: 10.1016/j.biopsych.2021.01.007. Epub 2021 Jan 21. Biol Psychiatry. 2021. PMID: 33726937 Free PMC article.
-
Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress?BMC Biol. 2006 Apr 13;4:8. doi: 10.1186/1741-7007-4-8. BMC Biol. 2006. PMID: 16613600 Free PMC article.
-
Optogenetic Central Amygdala Stimulation Intensifies and Narrows Motivation for Cocaine.J Neurosci. 2017 Aug 30;37(35):8330-8348. doi: 10.1523/JNEUROSCI.3141-16.2017. Epub 2017 Jul 27. J Neurosci. 2017. PMID: 28751460 Free PMC article.
-
Incentive motivation: 'wanting' roles of central amygdala circuitry.Behav Brain Res. 2021 Aug 6;411:113376. doi: 10.1016/j.bbr.2021.113376. Epub 2021 May 20. Behav Brain Res. 2021. PMID: 34023307 Free PMC article. Review.
-
Corticotropin releasing factor and neuroplasticity in cocaine addiction.Life Sci. 2010 Jan 2;86(1-2):1-9. doi: 10.1016/j.lfs.2009.11.005. Epub 2009 Nov 13. Life Sci. 2010. PMID: 19914260 Review.
Cited by
-
The Incentive-Sensitization Theory of Addiction 30 Years On.Annu Rev Psychol. 2025 Jan;76(1):29-58. doi: 10.1146/annurev-psych-011624-024031. Epub 2024 Dec 3. Annu Rev Psychol. 2025. PMID: 39094061 Free PMC article. Review.
-
Encoding and context-dependent control of reward consumption within the central nucleus of the amygdala.iScience. 2024 Apr 1;27(5):109652. doi: 10.1016/j.isci.2024.109652. eCollection 2024 May 17. iScience. 2024. PMID: 38650988 Free PMC article.
-
Distinct populations of corticotropin-releasing factor (CRF) neurons mediate divergent yet complementary defensive behaviors in response to a threat.Neuropharmacology. 2023 May 1;228:109461. doi: 10.1016/j.neuropharm.2023.109461. Epub 2023 Feb 10. Neuropharmacology. 2023. PMID: 36775096 Free PMC article. Review.
-
Neuromodulation of safety and surprise in the early stages of infant development: affective homeostatic regulation in bodily and mental functions.Front Psychol. 2024 Jun 6;15:1395247. doi: 10.3389/fpsyg.2024.1395247. eCollection 2024. Front Psychol. 2024. PMID: 38903479 Free PMC article. Review.
-
Corticotropin-Releasing Factor in the Nucleus Accumbens Does Not Drive High Levels of Cocaine Consumption.bioRxiv [Preprint]. 2025 Feb 26:2025.02.25.640140. doi: 10.1101/2025.02.25.640140. bioRxiv. 2025. PMID: 40060554 Free PMC article. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

