Isoflurane Disrupts Postsynaptic Density-95 Protein Interactions Causing Neuronal Synapse Loss and Cognitive Impairment in Juvenile Mice via Canonical NO-mediated Protein Kinase-G Signaling
- PMID: 35504002
- PMCID: PMC9332139
- DOI: 10.1097/ALN.0000000000004264
Isoflurane Disrupts Postsynaptic Density-95 Protein Interactions Causing Neuronal Synapse Loss and Cognitive Impairment in Juvenile Mice via Canonical NO-mediated Protein Kinase-G Signaling
Abstract
Background: Inhalational anesthetics are known to disrupt PDZ2 domain-mediated protein-protein interactions of the postsynaptic density (PSD)-95 protein. The aim of this study is to investigate the underlying mechanisms in response to early isoflurane exposure on synaptic PSD-95 PDZ2 domain disruption that altered spine densities and cognitive function. The authors hypothesized that activation of protein kinase-G by the components of nitric oxide (NO) signaling pathway constitutes a mechanism that prevents loss of early dendritic spines and synapse in neurons and cognitive impairment in mice in response to disruption of PDZ2 domain of the PSD-95 protein.
Methods: Postnatal day 7 mice were exposed to 1.5% isoflurane for 4 h or injected with 8 mg/kg active PSD-95 wild-type PDZ2 peptide or soluble guanylyl cyclase activator YC-1 along with their respective controls. Primary neurons at 7 days in vitro were exposed to isoflurane or PSD-95 wild-type PDZ2 peptide for 4 h. Coimmunoprecipitation, spine density, synapses, cyclic guanosine monophosphate-dependent protein kinase activity, and novel object recognition memory were assessed.
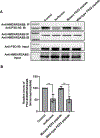
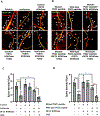
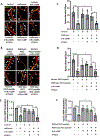
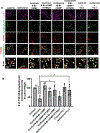
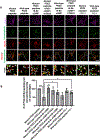
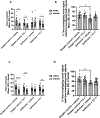
Results: Exposure of isoflurane or PSD-95 wild-type PDZ2 peptide relative to controls causes the following. First, there is a decrease in PSD-95 coimmunoprecipitate relative to N-methyl-d-aspartate receptor subunits NR2A and NR2B precipitate (mean ± SD [in percentage of control]: isoflurane, 54.73 ± 16.52, P = 0.001; and PSD-95 wild-type PDZ2 peptide, 51.32 ± 12.93, P = 0.001). Second, there is a loss in spine density (mean ± SD [spine density per 10 µm]: control, 5.28 ± 0.56 vs. isoflurane, 2.23 ± 0.67, P < 0.0001; and PSD-95 mutant PDZ2 peptide, 4.74 ± 0.94 vs. PSD-95 wild-type PDZ2 peptide, 1.47 ± 0.87, P < 0.001) and a decrease in synaptic puncta (mean ± SD [in percentage of control]: isoflurane, 41.1 ± 14.38, P = 0.001; and PSD-95 wild-type PDZ2 peptide, 50.49 ± 14.31, P < 0.001). NO donor or cyclic guanosine monophosphate analog prevents the spines and synapse loss and decline in the cyclic guanosine monophosphate-dependent protein kinase activity, but this prevention was blocked by soluble guanylyl cyclase or protein kinase-G inhibitors in primary neurons. Third, there were deficits in object recognition at 5 weeks (mean ± SD [recognition index]: male, control, 64.08 ± 10.57 vs. isoflurane, 48.49 ± 13.41, P = 0.001, n = 60; and female, control, 67.13 ± 11.17 vs. isoflurane, 53.76 ± 6.64, P = 0.003, n = 58). Isoflurane-induced impairment in recognition memory was preventable by the introduction of YC-1.
Conclusions: Activation of soluble guanylyl cyclase or protein kinase-G prevents isoflurane or PSD-95 wild-type PDZ2 peptide-induced loss of dendritic spines and synapse. Prevention of recognition memory with YC-1, a NO-independent activator of guanylyl cyclase, supports a role for the soluble guanylyl cyclase mediated protein kinase-G signaling in countering the effects of isoflurane-induced cognitive impairment.
Copyright © 2022, the American Society of Anesthesiologists. All Rights Reserved.
Conflict of interest statement
Figures


References
-
- Davidson AJ, Sun LS: Clinical Evidence for Any Effect of Anesthesia on the Developing Brain. Anesthesiology 2018; 128: 840–853 - PubMed
-
- Walters JL, Paule MG: Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol 2017; 60: 2–23 - PubMed
-
- Disma N, O’Leary JD, Loepke AW, Brambrink AM, Becke K, Clausen NG, De Graaff JC, Liu F, Hansen TG, McCann ME, Salorio CF, Soriano S, Sun LS, Szmuk P, Warner DO, Vutskits L, Davidson AJ: Anesthesia and the developing brain: A way forward for laboratory and clinical research. Paediatr Anaesth 2018; 28: 758–763 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

