Peptide Amphiphile Supramolecular Nanofibers Designed to Target Abdominal Aortic Aneurysms
- PMID: 35504018
- PMCID: PMC9733406
- DOI: 10.1021/acsnano.1c06258
Peptide Amphiphile Supramolecular Nanofibers Designed to Target Abdominal Aortic Aneurysms
Abstract
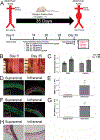
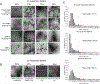
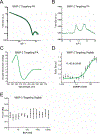
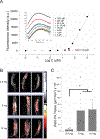
An abdominal aortic aneurysm (AAA) is a localized dilation of the aorta located in the abdomen that poses a severe risk of death when ruptured. The cause of AAA is not fully understood, but degradation of medial elastin due to elastolytic matrix metalloproteinases is a key step leading to aortic dilation. Current therapeutic interventions are limited to surgical repair to prevent catastrophic rupture. Here, we report the development of injectable supramolecular nanofibers using peptide amphiphile molecules designed to localize to AAA by targeting fragmented elastin, matrix metalloproteinase 2 (MMP-2), and membrane type 1 matrix metalloproteinase. We designed four targeting peptide sequences from X-ray crystallographic data and incorporated them into PA molecules via solid phase peptide synthesis. After coassembling targeted and diluent PAs at different molar ratios, we assessed their ability to form nanofibers using transmission electron microscopy and to localize to AAA in male and female Sprague-Dawley rats using light sheet fluorescence microscopy. We found that three formulations of the PA nanofibers were able to localize to AAA tissue, but the MMP-2 targeting PA substantially outperformed the other nanofibers. Additionally, we demonstrated that the MMP-2 targeting PA nanofibers had an optimal dose of 5 mg (∼12 mg/kg). Our results show that there was not a significant difference in targeting between male and female Sprague-Dawley rats. Given the ability of the MMP-2 targeting PA nanofiber to localize to AAA tissue, future studies will investigate potential diagnostic and targeted drug delivery applications for AAA.
Keywords: abdominal aortic aneurysm; matrix metalloproteinases; peptide amphiphile; self-assembly; targeted nanomaterials.
Figures


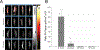
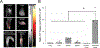
Similar articles
-
Continuous periaortic infusion improves doxycycline efficacy in experimental aortic aneurysms.J Vasc Surg. 2004 Jun;39(6):1312-21. doi: 10.1016/j.jvs.2004.01.036. J Vasc Surg. 2004. PMID: 15192574
-
Prevention of abdominal aortic aneurysm progression by targeted inhibition of matrix metalloproteinase activity with batimastat-loaded nanoparticles.Circ Res. 2015 Nov 6;117(11):e80-9. doi: 10.1161/CIRCRESAHA.115.307207. Epub 2015 Oct 6. Circ Res. 2015. PMID: 26443597 Free PMC article.
-
Cathepsin K-targeted sub-micron particles for regenerative repair of vascular elastic matrix.Acta Biomater. 2017 Apr 1;52:60-73. doi: 10.1016/j.actbio.2017.01.032. Epub 2017 Jan 10. Acta Biomater. 2017. PMID: 28087488 Free PMC article.
-
Plasminogen activators in abdominal aortic aneurysmal disease.Ann N Y Acad Sci. 1996 Nov 18;800:151-6. doi: 10.1111/j.1749-6632.1996.tb33306.x. Ann N Y Acad Sci. 1996. PMID: 8958990 Review.
-
Pentagalloyl Glucose and Its Functional Role in Vascular Health: Biomechanics and Drug-Delivery Characteristics.Ann Biomed Eng. 2019 Jan;47(1):39-59. doi: 10.1007/s10439-018-02145-5. Epub 2018 Oct 8. Ann Biomed Eng. 2019. PMID: 30298373 Free PMC article. Review.
Cited by
-
Therapeutic Supramolecular Polymers: Designs and Applications.Prog Polym Sci. 2024 Jan;148:101769. doi: 10.1016/j.progpolymsci.2023.101769. Epub 2023 Dec 2. Prog Polym Sci. 2024. PMID: 38188703 Free PMC article.
-
Combating Reactive Oxygen Species (ROS) with Antioxidant Supramolecular Polymers.ACS Appl Mater Interfaces. 2025 Jun 18;17(24):35275-35287. doi: 10.1021/acsami.5c06967. Epub 2025 Jun 3. ACS Appl Mater Interfaces. 2025. PMID: 40462549 Free PMC article.
-
Recent Advances in Nanomedicine-Mediated Abdominal Aortic Aneurysm Treatment.Small Methods. 2025 Jul;9(7):e2402268. doi: 10.1002/smtd.202402268. Epub 2025 May 19. Small Methods. 2025. PMID: 40384281 Free PMC article. Review.
-
Integrating Single-Walled Carbon Nanotubes into Supramolecular Assemblies: From Basic Interactions to Emerging Applications.ACS Nano. 2024 Oct 29;18(43):29380-29393. doi: 10.1021/acsnano.4c06843. Epub 2024 Oct 20. ACS Nano. 2024. PMID: 39428637 Free PMC article. Review.
-
Bioactive Supramolecular Polymers for Skin Regeneration Following Burn Injury.Biomacromolecules. 2025 Aug 11;26(8):5471-5482. doi: 10.1021/acs.biomac.5c01107. Epub 2025 Jul 16. Biomacromolecules. 2025. PMID: 40669455 Free PMC article.
References
-
- CDC. Aortic Aneurysm Fact Sheet. 2016. https://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_aortic_aneurysm... (accessed 2018 10/30).
-
- Keisler B; Carter C Abdominal Aortic Aneurysm. Am Fam Physician 2015, 91 (8), 538–543. - PubMed
-
- Argyriou C; Georgiadis GS; Kontopodis N; Pherwani AD; Van Herwaarden JA; Hazenberg C; Antoniou GA Screening for Abdominal Aortic Aneurysm During Transthoracic Echocardiography: A Systematic Review and Meta-Analysis. Eur J Vasc Endovasc Surg 2018, 55 (4), 475–491. DOI: 10.1016/j.ejvs.2018.01.003. - DOI - PubMed
-
- Upchurch GR Jr.; Schaub TA Abdominal Aortic Aneurysm. Am Fam Physician 2006, 73 (7), 1198–1204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

