Single-cell analysis of human testis aging and correlation with elevated body mass index
- PMID: 35504286
- PMCID: PMC9090997
- DOI: 10.1016/j.devcel.2022.04.004
Single-cell analysis of human testis aging and correlation with elevated body mass index
Abstract
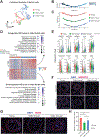
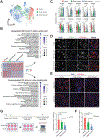
Aging men display reduced reproductive health; however, testis aging is poorly understood at the molecular and genomic levels. Here, we utilized single-cell RNA-seq to profile over 44,000 cells from both young and older men and examined age-related changes in germline development and in the testicular somatic cells. Age-related changes in spermatogonial stem cells appeared modest, whereas age-related dysregulation of spermatogenesis and somatic cells ranged from moderate to severe. Altered pathways included signaling and inflammation in multiple cell types, metabolic signaling in Sertoli cells, hedgehog signaling and testosterone production in Leydig cells, cell death and growth in testicular peritubular cells, and possible developmental regression in both Leydig and peritubular cells. Remarkably, the extent of dysregulation correlated with body mass index in older but not in younger men. Collectively, we reveal candidate molecular mechanisms underlying the complex testicular changes conferred by aging and their possible exacerbation by concurrent chronic conditions such as obesity.
Keywords: Leydig cell; Sertoli cell; male infertility; obesity; peritubular myoid cell; single-cell RNA sequencing; spermatogenesis; spermatogonial stem cell; testis aging; testis niche.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




References
-
- Albrecht M, Rämsch R, Köhn FM, Schwarzer JU, and Mayerhofer A (2006). Isolation and Cultivation of Human Testicular Peritubular Cells: A New Model for the Investigation of Fibrotic Processes in the Human Testis and Male Infertility. None 91, 1956–1960. - PubMed
-
- Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, Albrecht E, Amin N, Beekman M, de Geus EJC, et al. (2013). Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet 21, 1163–1168. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

