Oxygen-Free Days as an Outcome Measure in Clinical Trials of Therapies for COVID-19 and Other Causes of New-Onset Hypoxemia
- PMID: 35504307
- PMCID: PMC9055785
- DOI: 10.1016/j.chest.2022.04.145
Oxygen-Free Days as an Outcome Measure in Clinical Trials of Therapies for COVID-19 and Other Causes of New-Onset Hypoxemia
Abstract
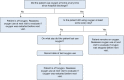
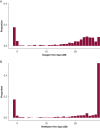
Mortality historically has been the primary outcome of choice for acute and critical care clinical trials. However, undue reliance on mortality can limit the scope of trials that can be performed. Large sample sizes are usually needed for trials powered for a mortality outcome, and focusing solely on mortality fails to recognize the importance that reducing morbidity can have on patients' lives. The COVID-19 pandemic has highlighted the need for rapid, efficient trials to rigorously evaluate new therapies for hospitalized patients with acute lung injury. Oxygen-free days (OFDs) is a novel outcome for clinical trials that is a composite of mortality and duration of new supplemental oxygen use. It is designed to characterize recovery from acute lung injury in populations with a high prevalence of new hypoxemia and supplemental oxygen use. In these populations, OFDs captures two patient-centered consequences of acute lung injury: mortality and hypoxemic lung dysfunction. Power to detect differences in OFDs typically is greater than that for other clinical trial outcomes, such as mortality and ventilator-free days. OFDs is the primary outcome for the Fourth Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-4) Host Tissue platform, which evaluates novel therapies targeting the host response to COVID-19 among adults hospitalized with COVID-19 and new hypoxemia. This article outlines the rationale for use of OFDs as an outcome for clinical trials, proposes a standardized method for defining and analyzing OFDs, and provides a framework for sample size calculations using the OFD outcome.
Keywords: COVID-19; acute lung injury; oxygen; respiratory failure.
Copyright © 2022 American College of Chest Physicians. All rights reserved.
Figures

Comment in
-
Clinical Recovery Should Be Considered as an Outcome Measure in Clinical Trials Including Patients With New-Onset Hypoxemia.Chest. 2023 Nov;164(5):e157-e158. doi: 10.1016/j.chest.2023.07.015. Epub 2023 Nov 7. Chest. 2023. PMID: 37945201 No abstract available.
-
Response.Chest. 2023 Nov;164(5):e158-e159. doi: 10.1016/j.chest.2023.07.016. Epub 2023 Nov 7. Chest. 2023. PMID: 37945202 No abstract available.
References
-
- Schoenfeld D.A., Bernard G.R. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

