Mouthrinses against SARS-CoV-2 - High antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial
- PMID: 35504446
- PMCID: PMC9057949
- DOI: 10.1016/j.virusres.2022.198791
Mouthrinses against SARS-CoV-2 - High antiviral effectivity by membrane disruption in vitro translates to mild effects in a randomized placebo-controlled clinical trial
Abstract
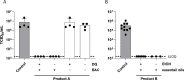
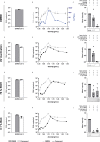
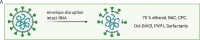
The emergence of the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) represents an unprecedented threat for the human population, necessitating rapid and effective intervention measures. Given the main infection route by airborne transmission, significant attention has been bestowed upon the use of antiseptic mouthrinses as a way to possibly reduce infectious viral titers. However, clinical evaluations are still sparse. Thus, we evaluated a wide variety of antiseptic agents that can be used as mouthrinses for their antiviral effects in vitro and their respective mode of action. One of the most promising antiseptic agents (benzalkoniumchloride, BAC) was used in a randomized placebo-controlled clinical trial with subsequent analysis of viral loads by RT-qPCR and virus rescue in cell culture. Mechanistic analysis revealed that treatment with BAC and other antiseptic agents efficiently inactivated SARS-CoV-2 in vitro by primarily disrupting the viral envelope, without affecting viral RNA integrity. However, the clinical application only resulted in a mild reduction of viral loads in the oral cavity. These results indicate that gargling with mouthrinses comprising single antiseptic agents may play a minor role towards a potential reduction of transmission rates and thus, these findings are of utmost importance when considering alternative COVID-19 prevention strategies.
Keywords: Antiseptic agents; Benzalkonium chloride; Capsid protection assay; Mouthrinse; Mouthwash; SARS-CoV-2.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

