Covid19 vaccination-associated portal vein thrombosis-An interdisciplinary clinical challenge
- PMID: 35504460
- PMCID: PMC9055786
- DOI: 10.1016/j.clinre.2022.101932
Covid19 vaccination-associated portal vein thrombosis-An interdisciplinary clinical challenge
Abstract
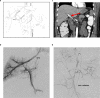
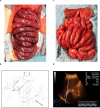
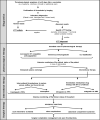
Despite one of the largest vaccination campaigns in human history, the COVID-19 pandemic has not been yet defeated. More than 10 billion doses of COVID-19 vaccine have been administered worldwide. AstraZeneca's Vaxzevria (ChAdOx1 nCoV-19 / AZD1222) was approved as the first viral vector-based vaccine in the EU on 29 January 2021. Thromboembolic events are a rare complication of vaccination with ChAdOx1 nCoV-19 in the context of, now known as vaccine-induced immune thrombotic thrombocytopenia (VITT), with an incidence of 1.5-3 in 100,000 vaccinations. VITT is clinically as well as pathophysiologically comparable to heparin-induced thrombocytopenia. Illustrated by a fulminant patient case, a multidisciplinary step-by-step guideline was developed for the recognition, diagnosis, and management of patients with severe acute portosplanchic venous thrombosis with mesenteric ischemia due to vaccine-induced immunogenic thrombotic thrombocytopenia.
Copyright © 2022 Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- WHO - COVID-19 Tools (ACT) Accelerator; Available from: https://www.who.int/initiatives/act-accelerator.
-
- WHO Coronavirus (COVID-19) Dashboard; Available from: https://covid19.who.int/.
-
- MacIntyre CR, Veness B, Berger D, Hamad N, Bari N. Thrombosis with Thrombocytopenia Syndrome (TTS) following AstraZeneca ChAdOx1 nCoV-19 (AZD1222) COVID-19 vaccination - A risk-benefit analysis for people < 60 years in Australia. Vaccine. 2021;39(34):4784–4787. doi: 10.1016/j.vaccine.2021.07.013. - DOI - PMC - PubMed
-
- National Institute for Health and Care Excellence. Covid-19 rapid guideline: vaccine-induced immune thrombocytopenia and thrombosis (VITT): NICE guideline [NG200], 2021. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

