TH17 cells promote CNS inflammation by sensing danger signals via Mincle
- PMID: 35504893
- PMCID: PMC9064974
- DOI: 10.1038/s41467-022-30174-1
TH17 cells promote CNS inflammation by sensing danger signals via Mincle
Abstract
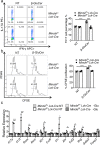
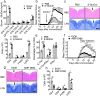
The C-type lectin receptor Mincle is known for its important role in innate immune cells in recognizing pathogen and damage associated molecular patterns. Here we report a T cell-intrinsic role for Mincle in the pathogenesis of experimental autoimmune encephalomyelitis (EAE). Genomic deletion of Mincle in T cells impairs TH17, but not TH1 cell-mediated EAE, in alignment with significantly higher expression of Mincle in TH17 cells than in TH1 cells. Mechanistically, dying cells release β-glucosylceramide during inflammation, which serves as natural ligand for Mincle. Ligand engagement induces activation of the ASC-NLRP3 inflammasome, which leads to Caspase8-dependent IL-1β production and consequentially TH17 cell proliferation via an autocrine regulatory loop. Chemical inhibition of β-glucosylceramide synthesis greatly reduces inflammatory CD4+ T cells in the central nervous system and inhibits EAE progression in mice. Taken together, this study indicates that sensing of danger signals by Mincle on TH17 cells plays a critical role in promoting CNS inflammation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review.
-
Simvastatin ameliorates experimental autoimmune encephalomyelitis by inhibiting Th1/Th17 response and cellular infiltration.Inflammopharmacology. 2015 Dec;23(6):343-54. doi: 10.1007/s10787-015-0252-1. Epub 2015 Nov 11. Inflammopharmacology. 2015. PMID: 26559850
-
LFA-1 Controls Th1 and Th17 Motility Behavior in the Inflamed Central Nervous System.Front Immunol. 2019 Oct 18;10:2436. doi: 10.3389/fimmu.2019.02436. eCollection 2019. Front Immunol. 2019. PMID: 31681316 Free PMC article.
-
Intracellular metabolite β-glucosylceramide is an endogenous Mincle ligand possessing immunostimulatory activity.Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):E3285-E3294. doi: 10.1073/pnas.1618133114. Epub 2017 Apr 3. Proc Natl Acad Sci U S A. 2017. PMID: 28373578 Free PMC article.
-
Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE).Brain Pathol. 2017 Mar;27(2):213-219. doi: 10.1111/bpa.12477. Brain Pathol. 2017. PMID: 27997058 Free PMC article. Review.
Cited by
-
Infiltrating myeloid cell-derived properdin markedly promotes microglia-mediated neuroinflammation after ischemic stroke.J Neuroinflammation. 2023 Nov 11;20(1):260. doi: 10.1186/s12974-023-02946-z. J Neuroinflammation. 2023. PMID: 37951917 Free PMC article.
-
The Dectin-1 and Dectin-2 clusters: C-type lectin receptors with fundamental roles in immunity.EMBO Rep. 2024 Dec;25(12):5239-5264. doi: 10.1038/s44319-024-00296-2. Epub 2024 Oct 31. EMBO Rep. 2024. PMID: 39482490 Free PMC article. Review.
-
The intracellular cation channel TMEM176B as a dual immunoregulator.Front Cell Dev Biol. 2022 Oct 20;10:1038429. doi: 10.3389/fcell.2022.1038429. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36340035 Free PMC article. Review.
-
Novel recombinant protein flagellin A N/C attenuates experimental autoimmune encephalomyelitis by suppressing the ROS/NF-κB/NLRP3 signaling pathway.Front Pharmacol. 2022 Nov 14;13:956402. doi: 10.3389/fphar.2022.956402. eCollection 2022. Front Pharmacol. 2022. PMID: 36452219 Free PMC article.
-
Glycolipids in Parkinson's disease: beyond neuronal function.FEBS Open Bio. 2023 Sep;13(9):1558-1579. doi: 10.1002/2211-5463.13651. Epub 2023 Jun 4. FEBS Open Bio. 2023. PMID: 37219461 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

