A human pancreatic ECM hydrogel optimized for 3-D modeling of the islet microenvironment
- PMID: 35504932
- PMCID: PMC9065104
- DOI: 10.1038/s41598-022-11085-z
A human pancreatic ECM hydrogel optimized for 3-D modeling of the islet microenvironment
Abstract
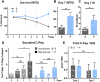
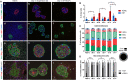
Extracellular matrix (ECM) plays a multitude of roles, including supporting cells through structural and biochemical interactions. ECM is damaged in the process of isolating human islets for clinical transplantation and basic research. A platform in which islets can be cultured in contact with natural pancreatic ECM is desirable to better understand and support islet health, and to recapitulate the native islet environment. Our study demonstrates the derivation of a practical and durable hydrogel from decellularized human pancreas that supports human islet survival and function. Islets embedded in this hydrogel show increased glucose- and KCl-stimulated insulin secretion, and improved mitochondrial function compared to islets cultured without pancreatic matrix. In extended culture, hydrogel co-culture significantly reduced levels of apoptosis compared to suspension culture and preserved controlled glucose-responsive function. Isolated islets displayed altered endocrine and non-endocrine cell arrangement compared to in situ islets; hydrogel preserved an islet architecture more similar to that observed in situ. RNA sequencing confirmed that gene expression differences between islets cultured in suspension and hydrogel largely fell within gene ontology terms related to extracellular signaling and adhesion. Natural pancreatic ECM improves the survival and physiology of isolated human islets.
© 2022. The Author(s).
Conflict of interest statement
The authors declare the following competing financial interest(s): JSO is scientific co-founder of Regenerative Medical Solutions, Inc. and has stock equity.
Figures




References
-
- Prince E, Kumacheva E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 2019;4:99–115. doi: 10.1038/s41578-018-0077-9. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

