Mutagenicity and safety pharmacology of a standardized antidiabetic polyherbal formulation
- PMID: 35505003
- PMCID: PMC9065066
- DOI: 10.1038/s41598-022-11243-3
Mutagenicity and safety pharmacology of a standardized antidiabetic polyherbal formulation
Abstract
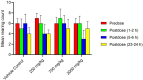
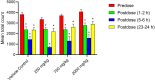
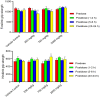
Synacinn is a standardized polyherbal extract formulated for the treatment of diabetes mellitus and its complications. This study aims to assess the mutagenicity potential of Synacinn by Ames assay and in vivo bone marrow micronucleus (MN) test on Sprague Dawley rat. Human ether-a-go-go-related gene (hERG) assay and Functional Observation Battery (FOB) were done for the safety pharmacology tests. In the Ames assay, Dose Range Finding (DRF) study and mutagenicity assays (+/- S9) were carried out. For the MN test, a preliminary and definitive study were conducted. In-life observations and number of immature and mature erythrocytes in the bone marrow cells were recorded. The hERG assay was conducted to determine the inhibitory effect on hERG potassium channel current expressed in human embryonic kidney cells (HEK293). FOB tests were performed orally (250, 750, and 2000 mg/kg) on Sprague Dawley rats. Synacinn is non-mutagenic against all tested strains of Salmonella typhimurium and did not induce any clastogenicity in the rat bone marrow. Synacinn also did not produce any significant inhibition (p ≤ 0.05) on hERG potassium current. Synacinn did not cause any neurobehavioural changes in rats up to 2000 mg/kg. Thus, no mutagenicity, cardiotoxicity and neurotoxicity effects of Synacinn were observed in this study.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Mutagenicity and genotoxicity effects of Verbena officinalis leaves extract in Sprague-Dawley Rats.J Ethnopharmacol. 2019 May 10;235:88-99. doi: 10.1016/j.jep.2019.02.007. Epub 2019 Feb 6. J Ethnopharmacol. 2019. PMID: 30738113
-
Genotoxicity and mutagenicity evaluation of isoquercitrin-γ-cyclodextrin molecular inclusion complex using Ames test and a combined micronucleus and comet assay in rats.J Toxicol Sci. 2022;47(6):221-235. doi: 10.2131/jts.47.221. J Toxicol Sci. 2022. PMID: 35650139
-
An oral toxicity test in rats and a genotoxicity study of extracts from the stems of Opuntia ficus-indica var. saboten.BMC Complement Altern Med. 2019 Jan 28;19(1):31. doi: 10.1186/s12906-019-2442-7. BMC Complement Altern Med. 2019. PMID: 30691445 Free PMC article.
-
Mutagenicity and clastogenicity evaluation of tacrine by Ames and micronucleus assays.Drug Chem Toxicol. 2012 Oct;35(4):366-70. doi: 10.3109/01480545.2011.627865. Epub 2011 Dec 19. Drug Chem Toxicol. 2012. PMID: 22182316
-
Synacinn™: Bacterial reverse mutation test data in five histidine-requiring strains of Salmonella Typhimurium.Data Brief. 2021 May 7;36:107075. doi: 10.1016/j.dib.2021.107075. eCollection 2021 Jun. Data Brief. 2021. PMID: 34041312 Free PMC article.
Cited by
-
In vitro and in silico evaluation of the design of nano-phyto-drug candidate for oral use against Staphylococcus aureus.PeerJ. 2023 Jun 8;11:e15523. doi: 10.7717/peerj.15523. eCollection 2023. PeerJ. 2023. PMID: 37309371 Free PMC article.
References
-
- Riet-Correa F, Medeiros RMT, Pfister JA, Mendonça FS. Toxic plants affecting the nervous system of ruminants and horses in Brazil. Pesquisa Vet. Brasil. 2017;37:1357–1368. doi: 10.1590/s0100-736x2017001200001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

