Nanoscale copper and silver thin film systems display differences in antiviral and antibacterial properties
- PMID: 35505071
- PMCID: PMC9063624
- DOI: 10.1038/s41598-022-11212-w
Nanoscale copper and silver thin film systems display differences in antiviral and antibacterial properties
Abstract
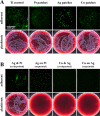
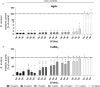
The current Coronavirus Disease 19 (COVID-19) pandemic has exemplified the need for simple and efficient prevention strategies that can be rapidly implemented to mitigate infection risks. Various surfaces have a long history of antimicrobial properties and are well described for the prevention of bacterial infections. However, their effect on many viruses has not been studied in depth. In the context of COVID-19, several surfaces, including copper (Cu) and silver (Ag) coatings have been described as efficient antiviral measures that can easily be implemented to slow viral transmission. In this study, we detected antiviral properties against Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) on surfaces, which were coated with Cu by magnetron sputtering as thin Cu films or as Cu/Ag ultrathin bimetallic nanopatches. However, no effect of Ag on viral titers was observed, in clear contrast to its well-known antibacterial properties. Further enhancement of Ag ion release kinetics based on an electrochemical sacrificial anode mechanism did not increase antiviral activity. These results clearly demonstrate that Cu and Ag thin film systems display significant differences in antiviral and antibacterial properties which need to be considered upon implementation.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

