Dietary intervention improves health metrics and life expectancy of the genetically obese Titan mouse
- PMID: 35505192
- PMCID: PMC9065075
- DOI: 10.1038/s42003-022-03339-3
Dietary intervention improves health metrics and life expectancy of the genetically obese Titan mouse
Abstract
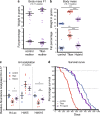
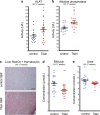
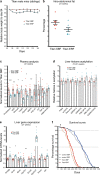
Suitable animal models are essential for translational research, especially in the case of complex, multifactorial conditions, such as obesity. The non-inbred mouse (Mus musculus) line Titan, also known as DU6, is one of the world's longest selection experiments for high body mass and was previously described as a model for metabolic healthy (benign) obesity. The present study further characterizes the geno- and phenotypes of this non-inbred mouse line and tests its suitability as an interventional obesity model. In contrast to previous findings, our data suggest that Titan mice are metabolically unhealthy obese and short-lived. Line-specific patterns of genetic invariability are in accordance with observed phenotypic traits. Titan mice also show modifications in the liver transcriptome, proteome, and epigenome linked to metabolic (dys)regulations. Importantly, dietary intervention partially reversed the metabolic phenotype in Titan mice and significantly extended their life expectancy. Therefore, the Titan mouse line is a valuable resource for translational and interventional obesity research.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

