Current and future colorectal cancer screening strategies
- PMID: 35505243
- PMCID: PMC9063618
- DOI: 10.1038/s41575-022-00612-y
Current and future colorectal cancer screening strategies
Erratum in
-
Author Correction: Current and future colorectal cancer screening strategies.Nat Rev Gastroenterol Hepatol. 2022 Aug;19(8):551. doi: 10.1038/s41575-022-00661-3. Nat Rev Gastroenterol Hepatol. 2022. PMID: 35787665 Free PMC article. No abstract available.
Abstract
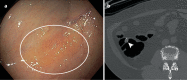
Despite strong evidence of effectiveness, colorectal cancer (CRC) screening remains underused. Currently, there are several options for CRC screening, each with its own performance characteristics and considerations for practice. This Review aims to cover current CRC screening guidelines and highlight future blood-based and imaging-based options for screening. In current practice, the leading non-invasive option is the faecal immunochemical test (FIT) based on its high specificity, good sensitivity, low cost and ease of use in mailed outreach programmes. There are currently five blood-based CRC screening tests in varying stages of evaluation, including one that is currently sold in the USA as a laboratory-developed test. There are ongoing studies on the diagnostic accuracy and longitudinal performance of blood tests and they have the potential to disrupt the CRC screening landscape. Imaging-based options, including the colon capsule, MR colonography and the CT capsule, are also being tested in active studies. As the world attempts to recover from the COVID-19 pandemic and adapts to the start of CRC screening among people at average risk starting at age 45 years, non-invasive options will become increasingly important.
© 2022. Springer Nature Limited.
Conflict of interest statement
A.S. has acted as a consultant for Freenome, Iterative Scopes and Medtronic. T.R.L. receives research funding from Freenome, is supported by The Permanente Medical Group, Delivery Science and Applied Research (TPMG DARE) Physician Researcher Program and is a board member of the California Colorectal Cancer Coalition.
Figures
References
-
- CDC Division of Cancer Prevention and Control CfDCaP. Colorectal Cancer Statistics (CDC, 2019).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

