Macroautophagy in CNS health and disease
- PMID: 35505254
- PMCID: PMC9282724
- DOI: 10.1038/s41583-022-00588-3
Macroautophagy in CNS health and disease
Abstract
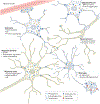
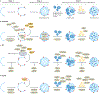
Macroautophagy is an evolutionarily conserved process that delivers diverse cellular contents to lysosomes for degradation. As our understanding of this pathway grows, so does our appreciation for its importance in disorders of the CNS. Once implicated primarily in neurodegenerative events owing to acute injury and ageing, macroautophagy is now also linked to disorders of neurodevelopment, indicating that it is essential for both the formation and maintenance of a healthy CNS. In parallel to understanding the significance of macroautophagy across contexts, we have gained a greater mechanistic insight into its physiological regulation and the breadth of cargoes it can degrade. Macroautophagy is a broadly used homeostatic process, giving rise to questions surrounding how defects in this single pathway could cause diseases with distinct clinical and pathological signatures. To address this complexity, we herein review macroautophagy in the mammalian CNS by examining three key features of the process and its relationship to disease: how it functions at a basal level in the discrete cell types of the brain and spinal cord; which cargoes are being degraded in physiological and pathological settings; and how the different stages of the macroautophagy pathway intersect with diseases of neurodevelopment and adult-onset neurodegeneration.
© 2022. Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

References
-
- Dixon JS “Phagocytic” lysosomes in chromatolytic neurones. Nature 215, 657–658 (1967). - PubMed
-
- De Duve C & Wattiaux R Functions of lysosomes. Annu. Rev. Physiol 28, 435–492 (1966). - PubMed
-
After discovering the lysosome, De Duve created the term ‘autophagy’ and, along with Novikoff and others, laid the foundation for the subsequent decades of study of this process in the CNS.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

