Toxic SOD1 trimers are off-pathway in the formation of amyloid-like fibrils in ALS
- PMID: 35505609
- PMCID: PMC9247475
- DOI: 10.1016/j.bpj.2022.04.037
Toxic SOD1 trimers are off-pathway in the formation of amyloid-like fibrils in ALS
Abstract
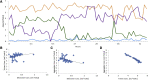
Accumulation of insoluble amyloid fibrils is widely studied as a critical factor in the pathology of multiple neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disease. Misfolded Cu, Zn superoxide dismutase (SOD1) was the first protein linked to ALS, and non-native SOD1 trimeric oligomers were recently linked to cytotoxicity, while larger oligomers were protective to cells. The balance between trimers and larger aggregates in the process of SOD1 aggregation is, thus, a critical determinant of potential therapeutic approaches to treat ALS. However, it is unknown whether these trimeric oligomers are a necessary intermediate for larger aggregate formation or a distinct off-pathway species competing with fibril formation. Depending on the on- or off-pathway scenario of trimer formation, we expect drastically different therapeutic approaches. Here, we show that the toxic SOD1 trimer is an off-pathway intermediate competing with protective fibril formation. We design mutant SOD1 constructs that remain in a trimeric state (super-stable trimers) and show that stabilizing the trimeric SOD1 prevents formation of fibrils in vitro and in a motor neuron-like cell model (NSC-34). Using size exclusion chromatography, we track the aggregation kinetics of purified SOD1 and show direct competition of trimeric SOD1 with larger oligomer and fibril formation. Finally, we show the trimer is structurally independent of both larger soluble oligomers and insoluble fibrils using circular dichroism spectroscopy and limited proteolysis.
Copyright © 2022 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

