Humoral immunity against SARS-CoV-2 variants including omicron in solid organ transplant recipients after three doses of a COVID-19 mRNA vaccine
- PMID: 35505864
- PMCID: PMC9052011
- DOI: 10.1002/cti2.1391
Humoral immunity against SARS-CoV-2 variants including omicron in solid organ transplant recipients after three doses of a COVID-19 mRNA vaccine
Abstract
Objectives: Solid organ transplant recipients (SOTR) receiving post-transplant immunosuppression show increased COVID-19-related mortality. It is unclear whether an additional dose of COVID-19 vaccines can overcome the reduced immune responsiveness against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants.
Methods: We analysed humoral immune responses against SARS-CoV-2 and its variants in 53 SOTR receiving SARS-CoV-2 vaccination.
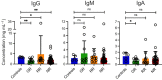
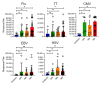
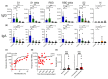
Results: Following the initial vaccination series, 60.3% of SOTR showed no measurable neutralisation and only 18.9% demonstrated neutralising activity of > 90%. More intensive immunosuppression, antimetabolites in particular, negatively impacted antiviral immunity. While absolute IgG levels were lower in SOTR than controls, antibody titres against microbial recall antigens were higher. By contrast, SOTR showed reduced vaccine-induced IgG/IgA antibody titres against SARS-CoV-2 and its delta variants and fewer linear B-cell epitopes, indicating reduced B-cell diversity. Importantly, a third vaccine dose led to an increase in anti-SARS-CoV-2 antibody titres and neutralising activity across alpha, beta and delta variants and to the induction of anti-SARS-CoV-2 CD4+ T cells in a subgroup of patients analysed. By contrast, we observed significantly lower antibody titres after the third dose with the omicron variant compared to the ancestral SARS-CoV-2 and the improvement in neutralising activity was much less pronounced than for all the other variants.
Conclusion: Only a small subgroup of solid organ transplant recipients is able to generate functional antibodies after an initial vaccine series; however, an additional vaccine dose resulted in dramatically improved antibody responses against all SARS-CoV-2 variants except omicron where antibody responses and neutralising activity remained suboptimal.
Keywords: COVID‐19; SARS‐CoV‐2; antibody responses; omicron variant; solid organ transplant; vaccine.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
