Identifying Signal-Crosstalk Mechanism in Maize Plants during Combined Salinity and Boron Stress Using Integrative Systems Biology Approaches
- PMID: 35505877
- PMCID: PMC9057046
- DOI: 10.1155/2022/1027288
Identifying Signal-Crosstalk Mechanism in Maize Plants during Combined Salinity and Boron Stress Using Integrative Systems Biology Approaches
Abstract
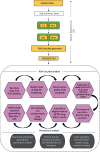
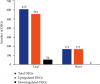
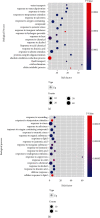
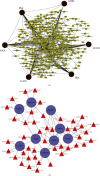
Combined stress has been seen as a major threat to world agriculture production. Maize is one of the leading cereal crops of the world due to its wide spectrum of growth conditions and is moderately sensitive to salt stress. A saline soil environment is a major factor that hinders its growth and overall yield and causes an increase in the concentration of micronutrients like boron, leading to excess over the requirement of the plant. Boron toxicity combined with salinity has been reported to be a serious threat to the yield and quality of maize. The response signatures of the maize plants to the combined effect of salinity and boron stress have not been studied well. We carried out an integrative systems-level analysis of the publicly available transcriptomic data generated on tolerant maize (Lluteño maize from the Atacama Desert, Chile) landrace under combined salt and boron stress. We identified significant biological processes that are differentially regulated in combined salt and boron stress in the leaves and roots of maize, respectively. Protein-protein interaction network analysis identified important roles of aldehyde dehydrogenase (ALDH), galactinol synthase 2 (GOLS2) proteins of leaf and proteolipid membrane potential regulator (pmpm4), metallothionein lea protein group 3 (mlg3), and cold regulated 410 (COR410) proteins of root in salt tolerance and regulating boron toxicity in maize. Identification of transcription factors coupled with regulatory network analysis using machine learning approach identified a few heat shock factors (HSFs) and NAC (NAM (no apical meristem, Petunia), ATAF1-2 (Arabidopsis thaliana activating factor), and CUC2 (cup-shaped cotyledon, Arabidopsis)) family transcription factors (TFs) to play crucial roles in salt tolerance, maintaining reactive oxygen species (ROS) levels and minimizing oxidative damage to the cells. These findings will provide new ways to design targeted functional validation experiments for developing multistress-resistant maize crops.
Copyright © 2022 Drishtee Barua et al.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this article.
Figures


References
-
- Laborde D., Martin W. Poverty and food insecurity could grow dramatically as COVID-19 spreads. In: Swinnen J., McDermott J., editors. COVID-19 and global food security . Washington DC: International Food Policy Research Institure (IFPRI); 2020. pp. 16–19.
-
- von Grebmer K., Bernstein J., Delgado C., et al. Map in 2021 Global Hunger Index: Hunger and Food Systems in Conflict Settings . Bonn: Welthungerhilfe; Dublin: Concern Worldwide; 2021. Figure 1.6: 2021 Global hunger index by severity.
-
- Bushra P., Sharma P. Climate change and its impacts on Indian agriculture: an econometric analysis. Journal of Public Affairs . 2020;20(1, article e1972) doi: 10.1002/pa.1972. - DOI
-
- Luck J., Spackman M., Freeman A., et al. Climate change and diseases of food crops. Plant Pathology . 2011;60(1):113–121. doi: 10.1111/j.1365-3059.2010.02414.x. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

