Pulsatile pulmonary artery pressure in a large animal model of chronic thromboembolic pulmonary hypertension: Similarities and differences with human data
- PMID: 35506099
- PMCID: PMC9052967
- DOI: 10.1002/pul2.12017
Pulsatile pulmonary artery pressure in a large animal model of chronic thromboembolic pulmonary hypertension: Similarities and differences with human data
Abstract
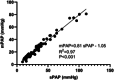
A striking feature of the human pulmonary circulation is that mean (mPAP) and systolic (sPAP) pulmonary artery pressures (PAPs) are strongly related and, thus, are essentially redundant. According to the empirical formula documented under normotensive and hypertensive conditions (mPAP = 0.61 sPAP + 2 mmHg), sPAP matches ~160%mPAP on average. This attests to the high pulsatility of PAP, as also witnessed by the near equality of PA pulse pressure and mPAP. Our prospective study tested if pressure redundancy and high pulsatility also apply in a piglet model of chronic thromboembolic pulmonary hypertension (CTEPH). At baseline (Week-0, W0), Sham (n = 8) and CTEPH (n = 27) had similar mPAP and stroke volume. At W6, mPAP increased in CTEPH only, with a two- to three-fold increase in PA stiffness and total pulmonary resistance. Seven CTEPH piglets were also studied at W16 at baseline, after volume loading, and after acute pulmonary embolism associated with dobutamine infusion. There was a strong linear relationship between sPAP and mPAP (1) at W0 and W6 (n = 70 data points, r² = 0.95); (2) in the subgroup studied at W16 (n = 21, r² = 0.97); and (3) when all data were pooled (n = 91, r² = 0.97, sPAP range 9-112 mmHg). The PA pulsatility was lower than that expected based on observations in humans: sPAP matched ~120%mPAP only and PA pulse pressure was markedly lower than mPAP. In conclusion, the redundancy between mPAP and sPAP seems a characteristic of the pulmonary circulation independent of the species. However, it is suggested that the sPAP thresholds used to define PH in animals are species- and/or model-dependent and thus must be validated.
Keywords: animal models; pathophysiology; pulmonary hypertension experimental; right ventricle function and dysfunction.
© 2021 The Authors. Pulmonary Circulation published by Wiley Periodicals LLC on behalf of the Pulmonary Vascular Research Institute.
Conflict of interest statement
The authors declare that there are no conflicts of interest in the field (physiology).
Figures
References
-
- Guihaire J, Bogaard HJ, Flécher E, Noly PE, Mercier O, Haddad F, Fadel E. Experimental models of right heart failure: a window for translational research in pulmonary hypertension. Semin Respir Crit Care Med. 2013;34:689–99. - PubMed
-
- Sztuka K, Jasińska‐Stroschein M. Animal models of pulmonary arterial hypertension: a systematic review and meta‐analysis of data from 6126 animals. Pharmacol Res. 2017;125:201–14. - PubMed
-
- Provencher S, Archer SL, Ramirez FD, Hibbert B, Paulin R, Boucherat O, Lacasse Y, Bonnet S. Standards and methodological rigor in pulmonary arterial hypertension preclinical and translational research. Circ Res. 2018;122:1021–32. - PubMed
-
- Lahm T, Douglas IS, Archer SL, Bogaard HJ, Chesler NC, Haddad F, Hemnes AR, Kawut SM, Kline JA, Kolb TM, Mathai SC, Mercier O, Michelakis ED, Naeije R, Tuder RM, Ventetuolo CE, Vieillard‐Baron A, Voelkel NF, Vonk‐Noordegraaf A, Hassoun PM, American Thoracic Society Assembly on Pulmonary Circulation . Assessment of right ventricular function in the research setting: knowledge gaps and pathways forward. An official American Thoracic Society research statement. Am J Respir Crit Care Med. 2018;198:e15–43. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

