A phase I study to evaluate safety, pharmacokinetics, and pharmacodynamics of respiratory syncytial virus neutralizing monoclonal antibody MK-1654 in healthy Japanese adults
- PMID: 35506164
- PMCID: PMC9283748
- DOI: 10.1111/cts.13290
A phase I study to evaluate safety, pharmacokinetics, and pharmacodynamics of respiratory syncytial virus neutralizing monoclonal antibody MK-1654 in healthy Japanese adults
Abstract
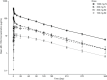
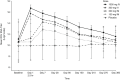
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract infection among all infants worldwide and remains a significant cause of morbidity and mortality. To address this unmet medical need, MK-1654, a half-life extended RSV neutralizing monoclonal antibody, is in clinical development for the prevention of RSV disease in infants. This was a phase I, randomized, placebo-controlled, single-site, double-blind trial of MK-1654 in 44 healthy Japanese adults. The safety, tolerability, pharmacokinetics, antidrug antibodies (ADAs), and serum neutralizing antibody (SNA) titers against RSV were evaluated for 1 year after a single intramuscular (i.m.) or intravenous (i.v.) dose of MK-1654 or placebo in five groups (100 mg i.m., 300 mg i.m., 300 mg i.v., 1000 mg i.v., or placebo). MK-1654 was generally well-tolerated in Japanese adults. There were no serious drug-related adverse events (AEs) reported in any MK-1654 recipient and no discontinuations due to any AEs in the study. The half-life of MK-1654 ranged from 76 to 91 days across dosing groups. Estimated bioavailability was 86% for 100 mg i.m. and 77% for 300 mg i.m. One participant out of 33 (3.0%) developed detectable ADA with no apparent associated AEs. The RSV SNA titers increased in a dose-dependent manner among participants who received MK-1654. These data support the development of MK-1654 for use in Japanese infants.
© 2022 Merck Sharp & Dohme LLC. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
Y.O., Y.M., K.F., N.O., B.M.M., L.C., and A.O.A. are employees (or were at the time of the study) of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may hold stock in Merck & Co., Inc., Rahway, NJ, USA. K.S.C. is an employee of Merck Sharp & Dohme LLC., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, may hold stock in Merck & Co., Inc., Rahway, NJ, USA, and is named on an issued patent related to the discovery of the RSV antibody. N.U. reports payments as a Primary Investigator for the study, is a paid consultant to MSD K.K. for general clinical pharmacology matters at the time of the conduct study, and holds stock in Merck & Co., Inc., Rahway, NJ, USA. All other authors declared no competing interest for this work. As an Associate Editor for
Figures
References
-
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among us infants, 1997 to 2000. J Pediatr. 2003;143:S127‐S132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

