Successful use of empagliflozin to treat neutropenia in two G6PC3-deficient children: Impact of a mutation in SGLT5
- PMID: 35506446
- PMCID: PMC9540799
- DOI: 10.1002/jimd.12509
Successful use of empagliflozin to treat neutropenia in two G6PC3-deficient children: Impact of a mutation in SGLT5
Abstract
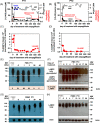
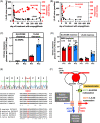
Neutropenia and neutrophil dysfunction found in deficiencies in G6PC3 and in the glucose-6-phosphate transporter (G6PT/SLC37A4) are due to accumulation of 1,5-anhydroglucitol-6-phosphate (1,5-AG6P), an inhibitor of hexokinase made from 1,5-anhydroglucitol (1,5-AG), an abundant polyol present in blood. Lowering blood 1,5-AG with an SGLT2 inhibitor greatly improved neutrophil counts and function in G6PC3-deficient mice and in patients with G6PT-deficiency. We evaluate this treatment in two G6PC3-deficient children. While neutropenia was severe in one child (PT1), which was dependent on granulocyte cololony-stimulating factor (GCSF), it was significantly milder in the other one (PT2), which had low blood 1,5-AG levels and only required GCSF during severe infections. Treatment with the SGLT2-inhibitor empagliflozin decreased 1,5-AG in blood and 1,5-AG6P in neutrophils and improved (PT1) or normalized (PT2) neutrophil counts, allowing to stop GCSF. On empagliflozin, both children remained infection-free (>1 year - PT2; >2 years - PT1) and no side effects were reported. Remarkably, sequencing of SGLT5, the gene encoding the putative renal transporter for 1,5-AG, disclosed a rare heterozygous missense mutation in PT2, replacing the extremely conserved Arg401 by a histidine. The higher urinary clearance of 1,5-AG explains the more benign neutropenia and the outstanding response to empagliflozin treatment found in this child. Our data shows that SGLT2 inhibitors are an excellent alternative to treat the neutropenia present in G6PC3-deficiency.
Keywords: 1,5-anhydroglucitol; G6PC3-deficiency; GSD1b; SGLT2-inhibitors; SGLT5; empagliflozin; glycogen Storage Disease 1b; neutropenia.
© 2022 The Authors. Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

