Bendamustine: A review of pharmacology, clinical use and immunological effects (Review)
- PMID: 35506458
- PMCID: PMC9100486
- DOI: 10.3892/or.2022.8325
Bendamustine: A review of pharmacology, clinical use and immunological effects (Review)
Abstract
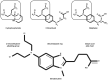
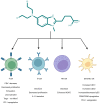
Bendamustine is an alkylating agent classified into the group of nitrogen mustard analogues, synthesized almost sixty years ago. It was registered in former East Germany in 1971 and approved by the US Food and Drug Administration in 2008 for treatment of chronic lymphocytic leukemia and indolent B‑cell non‑Hodgkin lymphoma. Considering its beneficial properties in the therapy of relapsed or refractory hematological malignancies, synergistic effects with other antineoplastic agents and increasing recent reports on its immunomodulatory effects, bendamustine has once again gained its justified attention. The uniqueness of bendamustine‑mediated effects should be observed keeping in mind its distinctive structure with structural similarities to both alkylating agents and purine analogs. In the present review, the current knowledge on the use of bendamustine in oncology, its pharmacokinetics, mechanism of action and toxicity was summarized. In addition, its immune‑modulating effects that have not been fully elucidated so far are emphasized, hoping to encourage further investigations of this unique drug.
Keywords: B‑cell; B‑cell chronic lymphocytic leukemia; T‑cell; bendamustine hydrochloride; cell cycle; cell death; immunomodulation; lymphocytes; non‑Hodgkin lymphoma.
Conflict of interest statement
IA received financial support from Roche, Takeda, Janssen, Novartis/Sandoz, AbbVie, Oktal Pharma/Celltrion, SanofiGenzyme, Alvogen, Teva/Pliva, and BMS. The remaining authors declare that they have no competing interests.
Figures

References
-
- WHO collaborating centre for drug statistics methodology, corp-author. ATC classification index with DDDs. Oslo, Norway: 2021.
-
- Cheson BD, Leoni L. Bendamustine: Mechanism of action and clinical data. Clin Adv Hematol Oncol. 2011;9((8 Suppl 19)):S1–S11. - PubMed
-
- World Health Organization model list of essential medicines, 21st list 2019, corp-author. Geneva: World Health Organization; 2019.

