Plicidentine in the oral fangs of parrotfish (Scarinae, Labriformes)
- PMID: 35506616
- PMCID: PMC9358764
- DOI: 10.1111/joa.13673
Plicidentine in the oral fangs of parrotfish (Scarinae, Labriformes)
Abstract
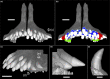
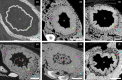
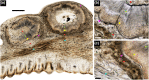
Parrotfish play important ecological roles in coral reef and seagrass communities across the globe. Their dentition is a fascinating object of study from an anatomical, functional and evolutionary point of view. Several species maintained non-interlocked dentition and browse on fleshy algae, while others evolved a characteristic beak-like structure made of a mass of coalesced teeth that they use to scrape or excavate food off hard limestone substrates. While parrotfish use their highly specialized marginal teeth to procure their food, they can also develop a series of large fangs that protrude from the upper jaw, and more rarely from the lower jaw. These peculiar fangs do not participate in the marginal dentition and their function remains unclear. Here we describe the morphology of these fangs and their developmental relationship to the rest of the oral dentition in the marbled parrotfish (Leptoscarus vaigiensis), the star-eye parrotfish (Calotomus carolinus), and the palenose parrotfish (Scarus psittacus). Through microtomographic and histological analyses, we show that some of these fangs display loosely folded plicidentine along their bases, a feature that has never been reported in parrotfish. Plicidentine is absent from the marginal teeth and is therefore exclusive to the fangs. Parrotfish fangs develop a particular type of simplexodont plicidentine with a pulpal infilling of alveolar bone at later stages of dental ontogeny. The occurrence of plicidentine and evidence of extensive tooth wear, and even breakage, lead us to conclude that the fangs undergo frequent mechanical stress, despite not being used to acquire food. This strong mechanical stress undergone by fangs could be linked either to forced contact with congeners or with the limestone substrate during feeding. Finally, we hypothesize that the presence of plicidentine in parrotfish is not derived from a labrid ancestor, but is probably a recently evolved trait in some parrotfish taxa, which may even have evolved convergently within this subfamily.
Keywords: Labridae; Scarinae; dentition; ecomorphology; evolution; functional anatomy; parrotfish; plicidentine.
© 2022 Anatomical Society.
Figures






Similar articles
-
Plicidentine and the repeated origins of snake venom fangs.Proc Biol Sci. 2021 Aug 11;288(1956):20211391. doi: 10.1098/rspb.2021.1391. Epub 2021 Aug 11. Proc Biol Sci. 2021. PMID: 34375553 Free PMC article.
-
Plicidentine in the Early Permian parareptile Colobomycter pholeter, and its phylogenetic and functional significance among coeval members of the clade.PLoS One. 2014 May 7;9(5):e96559. doi: 10.1371/journal.pone.0096559. eCollection 2014. PLoS One. 2014. PMID: 24804680 Free PMC article.
-
Influence of sexual selection and feeding functional morphology on diversification rate of parrotfishes (Scaridae).Proc Biol Sci. 2009 Oct 7;276(1672):3439-46. doi: 10.1098/rspb.2009.0876. Epub 2009 Jul 8. Proc Biol Sci. 2009. PMID: 19586949 Free PMC article.
-
The evolution of venom-conducting fangs: insights from developmental biology.Toxicon. 2007 Jun 1;49(7):975-81. doi: 10.1016/j.toxicon.2007.01.007. Epub 2007 Jan 26. Toxicon. 2007. PMID: 17337027 Review.
-
The genetic basis of modularity in the development and evolution of the vertebrate dentition.Philos Trans R Soc Lond B Biol Sci. 2001 Oct 29;356(1414):1633-53. doi: 10.1098/rstb.2001.0917. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11604128 Free PMC article. Review.
Cited by
-
De Novo Genome Assembly of the Whitespot Parrotfish (Scarus forsteni): A Valuable Scaridae Genomic Resource.Genes (Basel). 2024 Feb 17;15(2):249. doi: 10.3390/genes15020249. Genes (Basel). 2024. PMID: 38397238 Free PMC article.
-
Environmental sex reversal in parrotfish does not cause differences in the structure of their gut microbial communities.BMC Microbiol. 2024 Dec 19;24(1):531. doi: 10.1186/s12866-024-03698-3. BMC Microbiol. 2024. PMID: 39701987 Free PMC article.
References
-
- Agassiz, L. (1833. ‐1843) Recherches sur les Poissons Fossiles. Neuchâtel: Imprimerie de Petitpierre.
-
- Argyriou, T. , Giles, S. , Friedman, M. , Romano, C. , Kogan, I. & Sánchez‐Villagra, M.R. (2018) Internal cranial anatomy of Early Triassic species of Saurichthys (Actinopterygii: Saurichthyiformes): implications for the phylogenetic placement of saurichthyiforms. BMC Evolutionary Biology, 18, 161. - PMC - PubMed
-
- Baliga, V.B. & Law, C.J. (2016) Cleaners among wrasses: phylogenetics and evolutionary patterns of cleaning behavior within Labridae. Molecular Phylogenetics and Evolution, 94, 424–435. - PubMed
-
- Barlow, G. (1975) On the sociobiology of four Puerto Rican parrotfishes (Scaridae). Marine Biology, 33, 281–293.
-
- Bellwood, D.R. (1994) A phylogenetic study of the parrotfishes, family Scaridae (Pisces: Labroidei), with a revision of genera. Records of the Australian Museum Supplement, 20, 1–86.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

