Mechanisms Underlying Food-Triggered Symptoms in Disorders of Gut-Brain Interactions
- PMID: 35506862
- PMCID: PMC9169752
- DOI: 10.14309/ajg.0000000000001812
Mechanisms Underlying Food-Triggered Symptoms in Disorders of Gut-Brain Interactions
Abstract
There has been a dramatic increase in clinical studies examining the relationship between disorders of gut-brain interactions and symptoms evoked by food ingestion in the upper and lower gastrointestinal tract, but study design is challenging to verify valid endpoints. Consequently, mechanistic studies demonstrating biological relevance, biomarkers and novel therapeutic targets are greatly needed. This review highlights emerging mechanisms related to nutrient sensing and tasting, maldigestion, physical effects with underlying visceral hypersensitivity, allergy and immune mechanisms, food-microbiota interactions and gut-brain signaling, with a focus on patients with functional dyspepsia and irritable bowel syndrome. Many patients suffering from disorders of gut-brain interactions exhibit these mechanism(s) but which ones and which specific properties may vary widely from patient to patient. Thus, in addition to identifying these mechanisms and the need for further studies, biomarkers and novel therapeutic targets are identified that could enable enriched patient groups to be studied in future clinical trials examining the role of food in the generation of gut and non-gut symptoms.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of The American College of Gastroenterology.
Conflict of interest statement
Figures
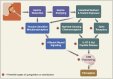
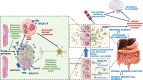

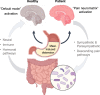
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

