Internal heating method of loop-mediated isothermal amplification for detection of HPV-6 DNA
- PMID: 35507110
- PMCID: PMC9065241
- DOI: 10.1007/s00604-022-05283-9
Internal heating method of loop-mediated isothermal amplification for detection of HPV-6 DNA
Abstract
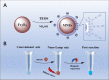
Loop-mediated isothermal amplification (LAMP) is a promising diagnostic tool for genetic amplification, which is known for its rapid process, simple operation, high amplification efficiency, and excellent sensitivity. However, most of the existing heating methods are external for completion of molecular amplification with possibility of contamination of specimens. The present research provided an internal heating method for LAMP using magnetic nanoparticles (MNPs), which is called nano-LAMP. Near-infrared light with an excitation wavelength of 808 nm was employed as the heating source; hydroxy naphthol blue (HNB) was used as an indicator to conduct methodological research. We demonstrate that the best temperature was controlled at a working power of 2 W and 4.8 µg/µL concentration of nanoparticles. The lowest limit for the detection of HPV by the nano-LAMP method is 102 copies/mL, which was confirmed by a gel electrophoresis assay. In the feasibility investigation of validated clinical samples, all 10 positive HPV-6 specimens amplified by nano-LAMP were consistent with conventional LAMP methods. Therefore, the nano-LAMP detection method using internal heating of MNPs may bring a new vision to the exploration of thermostatic detection in the future.
Keywords: Genetic amplification; Human papillomavirus; Loop-mediated isothermal amplification; Magnetic nanoparticles; Nano-LAMP.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Austria, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Dobec M, Bannwart F, Kaeppeli F, Cassinottiet P. Automation of the linear array HPV genotyping test and its application for routine typing of human papillomaviruses in cervical specimens of women without cytological abnormalities in Switzerland. J Clin Virol. 2009;45(1):23–27. doi: 10.1016/j.jcv.2009.03.005. - DOI - PubMed
-
- Maniar KP, Ronnett BM, Vang R, Yemelyanova A. Coexisting high-grade vulvar intraepithelial neoplasia (VIN) and condyloma acuminatum: independent lesions due to different HPV types occurring in immunocompromised patients. Am J Surg Pathol. 2013;37(1):53–60. doi: 10.1097/PAS.0b013e318263cda6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
