Immunogenicity and Safety of Standard and Third-Dose SARS-CoV-2 Vaccination in Patients Receiving Immunosuppressive Therapy
- PMID: 35507355
- PMCID: PMC9347774
- DOI: 10.1002/art.42153
Immunogenicity and Safety of Standard and Third-Dose SARS-CoV-2 Vaccination in Patients Receiving Immunosuppressive Therapy
Abstract
Objective: Immunogenicity and safety following receipt of the standard SARS-CoV-2 vaccination regimen in patients with immune-mediated inflammatory diseases (IMIDs) are poorly characterized, and data after receipt of the third vaccine dose are lacking. The aim of the study was to evaluate serologic responses and adverse events following the standard 2-dose regimen and a third dose of SARS-CoV-2 vaccine in IMID patients receiving immunosuppressive therapy.
Methods: Adult patients receiving immunosuppressive therapy for rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, Crohn's disease, or ulcerative colitis, as well as healthy adult controls, who received the standard 2-dose SARS-CoV-2 vaccination regimen were included in this prospective observational study. Analyses of antibodies to the receptor-binding domain (RBD) of the SARS-CoV-2 spike protein were performed prior to and 2-4 weeks after vaccination. Patients with a weak serologic response, defined as an IgG antibody titer of ≤100 arbitrary units per milliliter (AU/ml) against the receptor-binding domain of the full-length SARS-Cov-2 spike protein, were allotted a third vaccine dose.
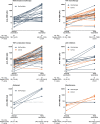
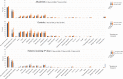
Results: A total of 1,505 patients (91%) and 1,096 healthy controls (98%) had a serologic response to the standard regimen (P < 0.001). Anti-RBD antibody levels were lower in patients (median 619 AU/ml interquartile range [IQR] 192-4,191) than in controls (median 3,355 AU/ml [IQR 896-7,849]) (P < 0.001). The proportion of responders was lowest among patients receiving tumor necrosis factor inhibitor combination therapy, JAK inhibitors, or abatacept. Younger age and receipt of messenger RNA-1273 vaccine were predictors of serologic response. Of 153 patients who had a weak response to the standard regimen and received a third dose, 129 (84%) became responders. The vaccine safety profile among patients and controls was comparable.
Conclusion: IMID patients had an attenuated response to the standard vaccination regimen as compared to healthy controls. A third vaccine dose was safe and resulted in serologic response in most patients. These data facilitate identification of patient groups at risk of an attenuated vaccine response, and they support administering a third vaccine dose to IMID patients with a weak serologic response to the standard regimen.
Trial registration: ClinicalTrials.gov NCT04798625.
© 2022 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures

References
-
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021;397:1819–29. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

