Visual cortex responds to sound onset and offset during passive listening
- PMID: 35507478
- PMCID: PMC9190746
- DOI: 10.1152/jn.00164.2021
Visual cortex responds to sound onset and offset during passive listening
Abstract
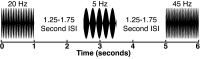
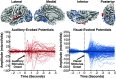
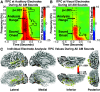
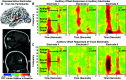
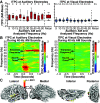
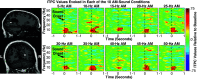
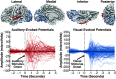
Sounds enhance our ability to detect, localize, and respond to co-occurring visual targets. Research suggests that sounds improve visual processing by resetting the phase of ongoing oscillations in visual cortex. However, it remains unclear what information is relayed from the auditory system to visual areas and if sounds modulate visual activity even in the absence of visual stimuli (e.g., during passive listening). Using intracranial electroencephalography (iEEG) in humans, we examined the sensitivity of visual cortex to three forms of auditory information during a passive listening task: auditory onset responses, auditory offset responses, and rhythmic entrainment to sounds. Because some auditory neurons respond to both sound onsets and offsets, visual timing and duration processing may benefit from each. In addition, if auditory entrainment information is relayed to visual cortex, it could support the processing of complex stimulus dynamics that are aligned between auditory and visual stimuli. Results demonstrate that in visual cortex, amplitude-modulated sounds elicited transient onset and offset responses in multiple areas, but no entrainment to sound modulation frequencies. These findings suggest that activity in visual cortex (as measured with iEEG in response to auditory stimuli) may not be affected by temporally fine-grained auditory stimulus dynamics during passive listening (though it remains possible that this signal may be observable with simultaneous auditory-visual stimuli). Moreover, auditory responses were maximal in low-level visual cortex, potentially implicating a direct pathway for rapid interactions between auditory and visual cortices. This mechanism may facilitate perception by time-locking visual computations to environmental events marked by auditory discontinuities.NEW & NOTEWORTHY Using intracranial electroencephalography (iEEG) in humans during a passive listening task, we demonstrate that sounds modulate activity in visual cortex at both the onset and offset of sounds, which likely supports visual timing and duration processing. However, more complex auditory rate information did not affect visual activity. These findings are based on one of the largest multisensory iEEG studies to date and reveal the type of information transmitted between auditory and visual regions.
Keywords: ECoG; amplitude modulation; audiovisual; multisensory; phase reset.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



References
-
- Stein BE, Meredith MA. The Merging of the Senses (1st ed.). Cambridge, MA: The MIT Press, 1993.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

