Efficacy and Safety of a Recombinant Plant-Based Adjuvanted Covid-19 Vaccine
- PMID: 35507508
- PMCID: PMC9127773
- DOI: 10.1056/NEJMoa2201300
Efficacy and Safety of a Recombinant Plant-Based Adjuvanted Covid-19 Vaccine
Abstract
Background: Coronavirus-like particles (CoVLP) that are produced in plants and display the prefusion spike glycoprotein of the original strain of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are combined with an adjuvant (Adjuvant System 03 [AS03]) to form the candidate vaccine.
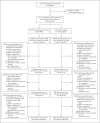
Methods: In this phase 3, multinational, randomized, placebo-controlled trial conducted at 85 centers, we assigned adults (≥18 years of age) in a 1:1 ratio to receive two intramuscular injections of the CoVLP+AS03 vaccine or placebo 21 days apart. The primary objective of the trial was to determine the efficacy of the CoVLP+AS03 vaccine in preventing symptomatic coronavirus disease 2019 (Covid-19) beginning at least 7 days after the second injection, with the analysis performed after the detection of at least 160 cases.
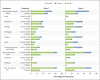
Results: A total of 24,141 volunteers participated in the trial; the median age of the participants was 29 years. Covid-19 was confirmed by polymerase-chain-reaction assay in 165 participants in the intention-to-treat population; all viral samples that could be sequenced contained variants of the original strain. Vaccine efficacy was 69.5% (95% confidence interval [CI], 56.7 to 78.8) against any symptomatic Covid-19 caused by five variants that were identified by sequencing. In a post hoc analysis, vaccine efficacy was 78.8% (95% CI, 55.8 to 90.8) against moderate-to-severe disease and 74.0% (95% CI, 62.1 to 82.5) among the participants who were seronegative at baseline. No severe cases of Covid-19 occurred in the vaccine group, in which the median viral load for breakthrough cases was lower than that in the placebo group by a factor of more than 100. Solicited adverse events were mostly mild or moderate and transient and were more frequent in the vaccine group than in the placebo group; local adverse events occurred in 92.3% and 45.5% of participants, respectively, and systemic adverse events in 87.3% and 65.0%. The incidence of unsolicited adverse events was similar in the two groups up to 21 days after each dose (22.7% and 20.4%) and from day 43 through day 201 (4.2% and 4.0%).
Conclusions: The CoVLP+AS03 vaccine was effective in preventing Covid-19 caused by a spectrum of variants, with efficacy ranging from 69.5% against symptomatic infection to 78.8% against moderate-to-severe disease. (Funded by Medicago; ClinicalTrials.gov number, NCT04636697.).
Copyright © 2022 Massachusetts Medical Society.
Figures



Comment in
-
Does the World Still Need New Covid-19 Vaccines?N Engl J Med. 2022 Jun 2;386(22):2140-2142. doi: 10.1056/NEJMe2204695. Epub 2022 May 4. N Engl J Med. 2022. PMID: 35507476 Free PMC article. No abstract available.
References
-
- WHO coronavirus disease (COVID-19) dashboard. 2021. (https://covid19.who.int/).
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
