Vocal changes in a zebra finch model of Parkinson's disease characterized by alpha-synuclein overexpression in the song-dedicated anterior forebrain pathway
- PMID: 35507553
- PMCID: PMC9067653
- DOI: 10.1371/journal.pone.0265604
Vocal changes in a zebra finch model of Parkinson's disease characterized by alpha-synuclein overexpression in the song-dedicated anterior forebrain pathway
Abstract
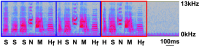
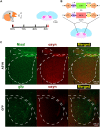
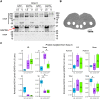
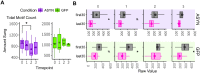
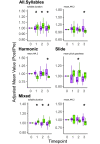
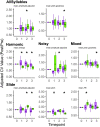
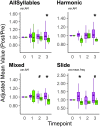
Deterioration in the quality of a person's voice and speech is an early marker of Parkinson's disease (PD). In humans, the neural circuit that supports vocal motor control consists of a cortico-basal ganglia-thalamo-cortico loop. The basal ganglia regions, striatum and globus pallidus, in this loop play a role in modulating the acoustic features of vocal behavior such as loudness, pitch, and articulatory rate. In PD, this area is implicated in pathogenesis. In animal models of PD, the accumulation of toxic aggregates containing the neuronal protein alpha-synuclein (αsyn) in the midbrain and striatum result in limb and vocal motor impairments. It has been challenging to study vocal impairments given the lack of well-defined cortico-basal ganglia circuitry for vocalization in rodent models. Furthermore, whether deterioration of voice quality early in PD is a direct result of αsyn-induced neuropathology is not yet known. Here, we take advantage of the well-characterized vocal circuits of the adult male zebra finch songbird to experimentally target a song-dedicated pathway, the anterior forebrain pathway, using an adeno-associated virus expressing the human wild-type αsyn gene, SNCA. We found that overexpression of αsyn in this pathway coincides with higher levels of insoluble, monomeric αsyn compared to control finches. Impairments in song production were also detected along with shorter and poorer quality syllables, which are the most basic unit of song. These vocal changes are similar to the vocal abnormalities observed in individuals with PD.
Conflict of interest statement
The authors have declared no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

