Caffeine Encapsulation in Metal Organic Framework MIL-53(Al) at Pilot Plant Scale for Preparation of Polyamide Textile Fibers with Cosmetic Properties
- PMID: 35507695
- PMCID: PMC9121351
- DOI: 10.1021/acsami.2c04293
Caffeine Encapsulation in Metal Organic Framework MIL-53(Al) at Pilot Plant Scale for Preparation of Polyamide Textile Fibers with Cosmetic Properties
Abstract
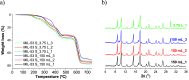
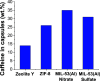
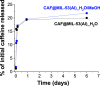
Currently in the marketplace, we can find clothing items able to release skin-friendly ingredients while wearing them. These innovative products with high-added value are based on microencapsulation technology. In this work, due to its lightness, flexibility, porosity, chemical affinity and adsorption capacity, metal-organic framework (MOF) MIL-53(Al) was the selected microcapsule to be synthesized at a large scale and subsequent caffeine encapsulation. The synthesis conditions (molar ratio of reactants, solvents used, reaction time, temperature, pressure reached in the reactor and activation treatment to enhance the encapsulation capacity) were optimized by screening various scaling-up reactor volumes (from lab-scale of 40 mL to pilot plant production of 3.75 L). Two types of Al salts (Al(NO3)3·9H2O from the original recipe and Al2(SO4)3 as commercial SUFAL 8.2) were employed. The liporeductor cosmetic caffeine was selected as the active molecule for encapsulation. Caffeine (38 wt %) was incorporated in CAF@MIL-53(Al) microcapsules, as analyzed by TGA and corroborated by GC/MS and UV-vis after additive extraction. CAF@MIL-53(Al) microcapsules showed a controlled release of caffeine during 6 days at 25 °C (up to 22% of the initial caffeine). These capsules were incorporated through an industrial spinning process (with temperatures up to 260 °C) to manufacture PA-6 fibers with cosmetic properties. Up to 0.7 wt % of capsules were successfully incorporated into the fibers hosting 1700 ppm of caffeine. Fabrics were submitted to scouring, staining, and washing processes, detecting the presence of caffeine in the cosmetic fiber.
Keywords: MIL-53(Al); Metal organic framework; caffeine; carboxylate ligand; microencapsulation; polyamide; scaled-up synthesis; textile fiber.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
-
- Valdes A.; Ramos M.; Beltran A.; Garrigos M. C. Recent Trends in Microencapsulation for Smart and Active Innovative Textile Products. Curr. Org. Chem. 2018, 22 (12), 1237–1248. 10.2174/1385272822666180430130528. - DOI
-
- Perez E.; Martin L.; Rubio C.; Urieta J. S.; Piera E.; Caballero M. A.; Tellez C.; Coronas J. Encapsulation of alpha-Tocopheryl Acetate into Zeolite Y for Textile Application. Ind. Eng. Chem. Res. 2010, 49 (18), 8495–8500. 10.1021/ie100483v. - DOI
-
- Metın D.; Tihminlioglu F.; Balkose D.; Ulku S. The Effect of Interfacial Interactions on the Mechanical Properties of Polypropylene/Natural Zeolite Composites. Composites Part A-Applied Science and Manufacturing 2004, 35 (1), 23–32. 10.1016/j.compositesa.2003.09.021. - DOI
-
- Earl D. J.; Deem M. W. Toward a Database of Hypothetical Zeolite Structures. Ind. Eng. Chem. Res. 2006, 45 (16), 5449–5454. 10.1021/ie0510728. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

