Maralixibat for the treatment of PFIC: Long-term, IBAT inhibition in an open-label, Phase 2 study
- PMID: 35507739
- PMCID: PMC9426380
- DOI: 10.1002/hep4.1980
Maralixibat for the treatment of PFIC: Long-term, IBAT inhibition in an open-label, Phase 2 study
Abstract
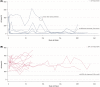
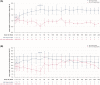
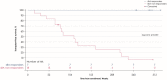
Children with progressive familial intrahepatic cholestasis, including bile salt export pump (BSEP) and familial intrahepatic cholestasis-associated protein 1 (FIC1) deficiencies, suffer debilitating cholestatic pruritus that adversely affects growth and quality of life (QoL). Reliance on surgical interventions, including liver transplantation, highlights the unmet therapeutic need. INDIGO was an open-label, Phase 2, international, long-term study to assess the efficacy and safety of maralixibat in children with FIC1 or BSEP deficiencies. Thirty-three patients, ranging from 12 months to 18 years of age, were enrolled. Eight had FIC1 deficiency and 25 had BSEP deficiency. Of the latter, 6 had biallelic, protein truncating mutations (t)-BSEP, and 19 had ≥ 1 nontruncating mutation (nt)-BSEP. Patients received maralixibat 266 μg/kg orally, once daily, from baseline to Week 72, with twice-daily dosing permitted from Week 72. Long-term efficacy was determined at Week 240. Serum bile acid (sBA) response (reduction in sBAs of > 75% from baseline or concentrations <102.0 μmol/L) was achieved in 7 patients with nt-BSEP, 6 during once-daily dosing, and 1 after switching to twice-daily dosing. sBA responders also demonstrated marked reductions in sBAs and pruritus, and increases in height, weight, and QoL. All sBA responders remained liver transplant-free after > 5 years. No patients with FIC1 deficiency or t-BSEP deficiency met the sBA responder criteria during the study. Maralixibat was generally well-tolerated throughout the study. Conclusion: Response to maralixibat was dependent on progressive familial intrahepatic cholestasis subtype, and 6 of 19 patients with nt-BSEP experienced rapid and sustained reductions in sBA levels. The 7 responders survived with native liver and experienced clinically significant reductions in pruritus and meaningful improvements in growth and QoL. Maralixibat may represent a well-tolerated alternative to surgical intervention.
Trial registration: ClinicalTrials.gov NCT02057718.
© 2022 The Authors. Hepatology Communications published by Wiley Periodicals LLC on behalf of American Association for the Study of Liver Diseases.
Conflict of interest statement
K.L. has received grants and consultancy fees from Mirum Pharmaceuticals and Albireo, and consultancy fees from Travere Therapeutics (formerly known as Retrophin). R.S. was a consultant for Mirum Pharmaceuticals. D.K has served as an advisor for Mirum Pharmaceuticals, Albireo, Astellas, and Intercept. N.S. has received grants from Mirum Pharmaceuticals and Albireo. C.M. has received consultancy fees from Albireo. K.S. has received consultancy fees from Mirum Pharmaceuticals and Retrophin, and is a stockholder in Asklepion Pharmaceuticals and Aliveris. A.D. and C.K. are shareholders in Mirum Pharmaceuticals. N.D is an employee and shareholder in Takeda Pharmaceuticals. P.V. and W.G. are stockholders in and employees of Mirum Pharmaceuticals. T.J. is a stockholder in and past employee of Mirum Pharmaceuticals, and is a stockholder of Vifor and Novartis Pharma. A.M. has received grants from the National Institutes of Health and has received consultancy fees from Mirum Pharmaceuticals and Metacrine. R.T. has received consultancy fees from Mirum Pharmaceuticals, Albireo, GenerationBio, Qing Bile Therapeutics, Alnylam, EVOX Therapeutics, Horizon Pharma, Rectify Therapeutics, and Sana Biotechnologies, and has stock options in Qing Bile Therapeutics, Rectify Therapeutics, and GenerationBio. All other authors declared no conflicts of interest.
Figures

References
-
- Baker A, Kerkar N, Todorova L, Kamath BM, Houwen RHJ. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2019;43:20–36. - PubMed
-
- Malatack JJ, Doyle D. A drug regimen for progressive familial cholestasis type 2. Pediatrics. 2018;141:e20163877. - PubMed
-
- van Wessel DBE, Thompson RJ, Gonzales E, Jankowska I, Sokal E, Grammatikopoulos T, et al. Genotype correlates with the natural history of severe bile salt export pump deficiency. J Hepatol. 2020;73:84–93. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

