Alterations in B- and circulating T-follicular helper cell subsets in immune thrombotic thrombocytopenic purpura
- PMID: 35507753
- PMCID: PMC9631570
- DOI: 10.1182/bloodadvances.2022007025
Alterations in B- and circulating T-follicular helper cell subsets in immune thrombotic thrombocytopenic purpura
Abstract
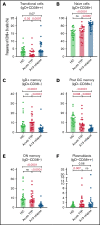
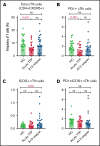
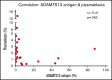
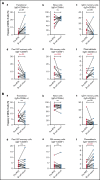
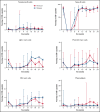
T follicular helper (Tfh) cells regulate development of antigen-specific B-cell immunity. We prospectively investigated B-cell and circulating Tfh (cTfh) cell subsets in 45 patients with immune thrombotic thrombocytopenic purpura (iTTP) at presentation and longitudinally after rituximab (RTX). B-cell phenotype was altered at acute iTTP presentation with decreased transitional cells and post-germinal center (post-GC) memory B cells and increased plasmablasts compared with healthy controls. A higher percentage of plasmablasts was associated with higher anti-ADAMTS13 IgG and lower ADAMTS13 antigen levels. In asymptomatic patients with ADAMTS13 relapse, there were increased naïve B cells and a global decrease in memory subsets, with a trend to increased plasmablasts. Total circulating Tfh (CD4+CXCR5+) and PD1+ Tfh cells were decreased at iTTP presentation. CD80 expression was decreased on IgD+ memory cells and double-negative memory cells in acute iTTP. At repopulation after B-cell depletion in de novo iTTP, post-GC and double-negative memory B cells were reduced compared with pre-RTX. RTX did not cause alteration in cTfh cell frequency. The subsequent kinetics of naïve, transitional, memory B cells and plasmablasts did not differ significantly between patients who went on to relapse vs those who remained in remission. In summary, acute iTTP is characterized by dysregulation of B- and cTfh cell homeostasis with depletion of post-GC memory cells and cTfh cells and increased plasmablasts. Changes in CD80 expression on B cells further suggest altered interactions with T cells.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures

References
-
- Kremer Hovinga JA, Coppo P, Lämmle B, Moake JL, Miyata T, Vanhoorelbeke K. Thrombotic thrombocytopenic purpura. Nat Rev Dis Primers. 2017;3(1):17020-17020. - PubMed
-
- Sorvillo N, van Haren SD, Kaijen PH, et al. . Preferential HLA-DRB1*11-dependent presentation of CUB2-derived peptides by ADAMTS13-pulsed dendritic cells. Blood. 2013;121(17):3502-3510. - PubMed
-
- Verbij FC, Turksma AW, de Heij F, et al. . CD4+ T cells from patients with acquired thrombotic thrombocytopenic purpura recognize CUB2 domain-derived peptides. Blood. 2016;127(12):1606-1609. - PubMed
-
- Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol Rev. 2004;197(1):179-191. - PubMed
-
- Westwood JP, Webster H, McGuckin S, McDonald V, Machin SJ, Scully M. Rituximab for thrombotic thrombocytopenic purpura: benefit of early administration during acute episodes and use of prophylaxis to prevent relapse. J Thromb Haemost. 2013;11(3):481-490. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

