Molecular Mechanisms of Iron and Heme Metabolism
- PMID: 35508203
- PMCID: PMC9398995
- DOI: 10.1146/annurev-nutr-062320-112625
Molecular Mechanisms of Iron and Heme Metabolism
Abstract
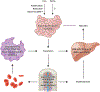
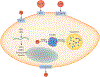
An abundant metal in the human body, iron is essential for key biological pathways including oxygen transport, DNA metabolism, and mitochondrial function. Most iron is bound to heme but it can also be incorporated into iron-sulfur clusters or bind directly to proteins. Iron's capacity to cycle between Fe2+ and Fe3+ contributes to its biological utility but also renders it toxic in excess. Heme is an iron-containing tetrapyrrole essential for diverse biological functions including gas transport and sensing, oxidative metabolism, and xenobiotic detoxification. Like iron, heme is essential yet toxic in excess. As such, both iron and heme homeostasis are tightly regulated. Here we discuss molecular and physiologic aspects of iron and heme metabolism. We focus on dietary absorption; cellular import; utilization; and export, recycling, and elimination, emphasizing studies published in recent years. We end with a discussion on current challenges and needs in the field of iron and heme biology.
Keywords: heme; iron; metabolism; porphyrin; tetrapyrrole; trafficking.
Figures

References
-
- Andolfo I, Rosato BE, Manna F, Rosa GD, Marra R, et al. 2020. Gain-of-function mutations in PIEZO1 directly impair hepatic iron metabolism via the inhibition of the BMP/SMADs pathway. Am. J. Hematol 95(2):188–97 - PubMed
-
- Ascenzi P, Bocedi A, Visca P, Altruda F, Tolosano E, et al. 2005. Hemoglobin and heme scavenging. IUBMB Life 57(11):749–59 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

