Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases
- PMID: 35508284
- PMCID: PMC9057012
- DOI: 10.1053/j.gastro.2022.04.037
Postacute COVID-19 is Characterized by Gut Viral Antigen Persistence in Inflammatory Bowel Diseases
Abstract
Background & aims: The coronavirus disease 2019 (COVID-19) pandemic has affected populations, societies, and lives for more than 2 years. Long-term sequelae of COVID-19, collectively termed the postacute COVID-19 syndrome, are rapidly emerging across the globe. Here, we investigated whether severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigen persistence underlies the postacute COVID-19 syndrome.
Methods: We performed an endoscopy study with 46 patients with inflammatory bowel disease (IBD) 219 days (range, 94-257) after a confirmed COVID-19 infection. SARS-CoV-2 antigen persistence was assessed in the small and large intestine using quantitative polymerase chain reaction of 4 viral transcripts, immunofluorescence of viral nucleocapsid, and virus cultivation from biopsy tissue. Postacute COVID-19 was assessed using a standardized questionnaire, and a systemic SARS-CoV-2 immune response was evaluated using flow cytometry and enzyme-linked immunosorbent assay at endoscopy. IBD activity was evaluated using clinical, biochemical, and endoscopic means.
Results: We report expression of SARS-CoV-2 RNA in the gut mucosa ∼7 months after mild acute COVID-19 in 32 of 46 patients with IBD. Viral nucleocapsid protein persisted in 24 of 46 patients in gut epithelium and CD8+ T cells. Expression of SARS-CoV-2 antigens was not detectable in stool and viral antigen persistence was unrelated to severity of acute COVID-19, immunosuppressive therapy, and gut inflammation. We were unable to culture SARS-CoV-2 from gut tissue of patients with viral antigen persistence. Postacute sequelae of COVID-19 were reported from the majority of patients with viral antigen persistence, but not from patients without viral antigen persistence.
Conclusion: Our results indicate that SARS-CoV-2 antigen persistence in infected tissues serves as a basis for postacute COVID-19. The concept that viral antigen persistence instigates immune perturbation and postacute COVID-19 requires validation in controlled clinical trials.
Keywords: COVID-19; Postacute COVID-19; SARS-CoV-2; Viral Antigen Persistence.
Copyright © 2022 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures






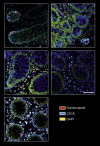
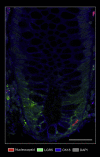
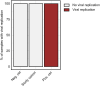


Comment in
-
Rear Window-What Can the Gut Tell Us About Long-COVID?Gastroenterology. 2022 Aug;163(2):376-378. doi: 10.1053/j.gastro.2022.05.044. Epub 2022 Jun 2. Gastroenterology. 2022. PMID: 35660031 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

